Abstract
Aims:
This study investigated the effects of indole-3-carbinol (I3C), a compound from cruciferous vegetables, on various parameters related to obesity, in particular, the parameters of infiltration by macrophages and of inflammatory cytokines expressed during the co-culture of adipocytes and macrophages.
Methods:
Male C57BL/6 mice were fed with a control diet (C group), high-fat diet (HF group) and HF+5 mg kg−1 I3C (HFI group). The I3C was intraperitoneally injected (HFI group) for 12 weeks. Epididymal adipose tissue (AT) was collected and stained for F4/80, a marker of macrophages.
Results:
The immunohistochemical staining for F4/80 indicated a greater presence of macrophages in the HF group than in AT from the control and HFI groups. Furthermore, I3C treatment, in an in vitro cell culture system, decreased expression of inducible nitric oxide synthase (iNOS), decreased nitrite content and enhanced expression of peroxisome proliferator-activated receptor (PPAR-γ). Moreover, in vitro cell culture studies revealed that I3C inhibited intracellular lipid accumulation in hypertrophied adipocytes. In macrophage and primary adipocyte co-culture, I3C inhibited expression of interleukin-6 (IL-6).
Conclusions:
In vivo treatment with I3C reduced the infiltration of macrophages in AT, and in vitro addition of I3C to co-cultured macrophages and adipocytes reduced nitrite production and IL-6 expression. With cultures of adipocytes only, I3C inhibited accumulation of intracellular lipid, either by disrupting differentiation, or by directly inhibiting triglyceride synthesis.
Keywords: Indole-3-carbinol, inflammation, macrophages, adipocytes
Introduction
Obesity is accompanied by chronic inflammation. Hyperplasia adipocytes will produce numerous mediators, including adipokines, associated with adipose tissue (AT) inflammation, as obesity can induce the release of inflammatory cytokines.1 Chronic inflammation can cause obesity2 and contributes to an increased risk of type 2 diabetes and atherosclerosis.3, 4, 5, 6 AT is composed of various cell types, including mature adipocytes and a stromal vascular fraction that includes blood cells, endothelial cells and macrophages.7 Adipocytes have recently been classified as members of the immune system, as these cells are potent producers of inflammatory cytokines, such as interleukin-6 (IL-6).8 Obesity is associated with an increase in number of macrophages in AT, where this increase in macrophages could represent either the cause or the consequence of the low-grade inflammation state associated with obesity.6, 9, 10 Macrophage infiltration is associated with the expression of proinflammatory adipocytokines in obese AT.11 Thus, obesity can be characterized as a disorder having an imbalance between pro-inflammatory and anti-inflammatory mediators, and by obesity-induced AT inflammation.12, 13 Adipocytes can express various cytokines, such as leptin, adiponectin, resistin, tumor necrosis factor-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1). When secreted by adipocytes and macrophages, these cytokines modulate inflammation in obese AT.14, 15 Tumor necrosis factor-α, IL-6 and MCP-1 are inflammatory cytokines that are commonly elevated in obesity.9 Therefore, interactions between macrophages and adipocytes may contribute to the inflammatory pathology found in obese AT.16 The following concerns nitric oxide synthase and nitrite. Chronic inflammation contributes to obesity-induced insulin resistance. Inducible nitric oxide synthase (iNOS), a mediator of inflammation, has recently been found to contribute to insulin resistance. Obesity is associated with increased iNOS expression in insulin-sensitive tissues in rodents and humans.17
Cruciferous vegetables contain a number of bioactive components including glucosinolates and indole-3-carbinol (I3C). Epidemiology studies showed that a higher consumption of cruciferous vegetables, for example, broccoli, cauliflower and brussels sprouts, diminishes the risk of cancer and cardiovascular diseases.18, 19Although cruciferous vegetables contain a number of compounds for cancer prevention, I3C alone shows efficacy for the prevention of breast, endometrial and cervical cancers.20, 21 We have demonstrated that I3C can suppress lipopolysaccharide-induced iNOS, nitric oxide, and proinflammatory cytokines in macrophages.22 We also demonstrated that intraperitoneally injected I3C reduces body weight in high-fat (HF) diet-induced obesity. The present study was aimed at investigating the effects of I3C on macrophage infiltration in obese AT, and on potential mechanism of inflammatory mediators during the in vitro co-culture of macrophages and adipocytes.
Materials and methods
Chemicals and biochemicals
I3C with 98% purity, lipopolysaccharide, sulfanilamide, naphthylethylenediamine dihydrochloride, sodium nitrite, insulin and collagenase were obtained from Sigma Chemical (St Louis, MO, USA). Absolute ethanol was from Merck (Darmstadt, Germany). Dulbecco's Modified Eagle Medium and fetal bovine serum were from GIBCO (Grand Island, NY, USA). I3C was dissolved in absolute ethanol. The concentration of I3C in media was 0.1% (v/v).
Animals and diets
Eighteen 5-week-old male C57BL/6 mice were obtained from the National Taiwan University Animal Center (Taipei, Taiwan), and were raised at 22±3 °C under automatic lighting cycles. After a 1-week acclimation period, mice were randomly assigned to three groups: control (C), high fat (HF) and HF+I3C (HFI). The composition of the diets was a modified version of the AIN-96 M diet used by Jiang et al.23 The HF diet contained 15.29% of soybean oil and 15.29% lard as fat (>55% of total energy), which induces hyperglycemia, hyperinsulinemia, hyperlipidemia and obesity in animals after 12 weeks of feeding.
I3C was administered intraperitoneally three times per week on the same weekdays at a dose of 5 mg kg−1 body weight in a 0.1-ml vehicle containing polyethylene glycol, benzyl alcohol, ethanol and water (40:0.1:10:49.9),24 and animal in both control and HF groups received vehicle injection only. At the end of the experiment (12 weeks), mice were sacrificed by cervical dislocation, and epididymal AT were collected for analysis.
I3C dosage considerations
The I3C dose selected for this study was derived from previous reports that recorded beneficial outcomes in mice with 5 mg kg−1, administered by intraperitoneal injection and without any apparent toxicity.25, 26 For a 60 kg human, a 5 mg kg−1 dose equates to a dietary intake of 300 mg I3C obtained by consuming 1800 g Chinese cabbage,27 without considering losses due to absorption and metabolism in the body. The clinical administration of I3C at a dosage of 300–400 mg daily has been shown to be a safe and effective chemopreventative oral supplement in females at risk of developing breast cancer.28, 29 We have, therefore, selected a dosage in mice that is known to be clinically relevant in humans. Although drug efficacy is dependent on the route of administration, mice showed protection against hormone-induced tumorigenesis when their diet was supplemented with 70 mg kg−1 I3C (equal to 500 ppm),30 14 times the intraperitoneal dosage applied here. This would suggest that 5 mg kg−1 is a very cautious dose.
Immunohistochemistry
Epididymal AT was fixed with 4% paraformaldehyde and excised, dehydrated in ethanol, and embedded in paraffin. For immunohistochemistry, the tissue was blocked with 3% H2O2 and sequentially incubated with anti-mouse F4/80 antibody (Serotec, Raleigh, NC, USA) at 1:100 dilution in 10% serum for 2 h, biotinylated anti-rat IgG (Vector Laboratories, Burlingame, CA, USA) at 1:200 in 10% serum for 1 h, and streptavidin horseradish peroxidase (Vector Laboratories, Burlingame, CA, USA) at 1:500 in phosphate-buffered saline for 20 min, with phosphate-buffered saline washes after each incubation. Sections were incubated with the 3,3′-diaminobenzidine substrate kit (Vector Laboratories, Burlingame, CA, USA). The tissue was stained with dilute hematoxylin and then was photographed (Nikon, Japan).
Preparation of primary adipocytes
C57BL/6 male mice (8–12 weeks of age) were anesthetized and sacrificed, and epididymal AT was collected. The tissues were cut and incubated with 2% collagenase for 30 min at 37 °C and passed through 40 μm filter. After centrifugation at 500 × g for 10 min, the cell pellet was washed twice with the culture medium. Cells were seeded at 1 × 106 ml−1 in each well.
Cell culture
RAW 264.7 cell lines (BCRC 60001) and 3T3-L1 preadipocytes (BCRC 60159) were purchased from Bioresource Collection and Research Center (Hsinchu, Taiwan). The 3T3-L1 cell line is widely used as a model of adipocyte differentiation and AT biology.31 Primary preadipocytes were obtained from epididymal AT of C57BL/6 mice. The cells were cultured in Dulbecco's modified essential medium containing 10% fetal bovine serum at 37 °C in 5% CO2. For induction of preadipocyte differentiation, primary preadipocytes were incubated in a medium containing insulin (INS), dexamethasone, and 3-isobutyl-1-methyl-xanthine as described previously.32 The preadipocytes were differentiated into lipid-laden mature adipocytes (primary adipocytes), and cultured up to the fourteenth day.
Adipocytes and macrophages co-culture model
Co-culture of primary adipocytes and macrophages was performed as described.14 In the co-culture model, macrophages (106 cells) were plated into hypertrophied primary adipocytes (106 cells). The cells were cultured for 24 h and treated with various concentrations of I3C for 24 h. The supernatants and total RNA of co-cultured cells were collected in each experiment.
Measurements of nitrite production
The nitrite concentration in the culture medium was determined as an index of nitric oxide production. Nitrite was quantified spectrophotometrically after its reaction with Griess reagent (1:1 mixture of 1% sulfanilamide/ 5% H3PO4 and 0.1% naphthylethylenediamine dihydrochloride) using sodium nitrite as a substrate.33
Measurement of IL-6
The culture medium concentration of IL-6 was measured with an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's protocols.
Reverse transcriptase polymerase chain reaction analysis
Reverse transcriptase polymerase chain reaction was used to determine the in vitro effects of I3C on the expression of lipogenesis-related genes. Total RNA was extracted from co-culture cells by using TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), according to the manufacturer's instructions. After the quality was validated by denaturing agarose gel, the RNA was subjected to reverse transcription to obtain cDNA. In brief, 2 μg total RNA was incubated with Super Script β (Invitrogen Life Technologies, Carlsbad, CA, USA), dNTP, oligo-dT, dithiothreitol and reaction buffer at 37 °C for 60 min. The obtained cDNA (2 μl) was then subjected to PCR, which consisted of denaturation at 94 °C for 45 s, annealing at 60 °C for 60 s and elongation at 72 °C for 60 s for 32 cycles. The last cycle was ended by 10 min of elongation at 72 °C. The following primers were used: beta-actin, 5′-AGCCATGTACGTAGCCATCC-3′ (forward), 5′-CTCTCAGCTGTGGTGGTGAA-3′ (reverse), 228 bp; peroxisome proliferator-activated receptor (PPAR-γ), 5′-GTTCATGCTTGTGAAGGATGC-3′(forward), and 5′-ACTCTGGGTTCAGCTGGTCG-3′ (reverse), 250 bp; iNOS, 5′-CCCTTCCGAAGTTTCTGGGAGGAGC-3′ (forward), 5′-GGCTGTCAGAGAGCCTCGTGGCTTTGG-3′ (reverse), 497 bp;. The PCR products were then separated by agarose gel, and the images were visualized and quantified by using the Image-pro Plus program (Media Cybernetics Inc., Bethesda, MD, USA).
Oil red O staining of 3T3-L1 adipocytes
The 3T3-L1 cell line is widely used as a model of adipocyte differentiation and adipose biology.31 These cells were exposed to various concentrations of I3C, with the goal of inducing differentiation to hypertrophied-3T3-L1 adipocytes. Intracellular lipid accumulation, which was a marker of adipocyte differentiation and adipogenesis, was determined by using oil red O. The oil red O dye was prepared as described by Ramirez-Zacaruas et al.34 Triglyceride concentrations were analyzed by using Randox TRIGS (Cat TG213) assay kits (Randox Labs, Crumlin, UK), and measured by using an automatic microplate reader (VersaMax, Sunnyvale, CA, USA).
Statistical analysis
All data are presented as mean±s.d. One-way analysis of variance was used to compare the differences in body weight, weight of organs and F4/80 marker expression in response to the various types of diet. Also, analysis of variance test was performed to confirm the differences in nitrite expression, level of IL-6 and triglyceride accumulation among four co-culture groups. When statistical results were revealed by analysis of variance, Tukey's post hoc test was used to determine post hoc differences. If the standard deviations for each group were not equal, Dunnett's T3 post hoc test was used. The difference between two non-co-culture group and co-culture group without I3C was verified with an independent t-test. Differences were considered statistically significant when P<0.05. All statistics were two-sided and performed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
Results
Effect of I3C on macrophage infiltration in AT, body weight and weight of organ
As Table 1 shows, regardless of type of diet, baseline body weight of rats in three groups was similar (P=0.984); however, the body weight at week 12 in the HF group was significantly higher than that in the control diet group (P<0.001), and the body weight at week 12 in the I3C-treated HF group (HFI) was significantly lower than that in the HF group (P<0.001). The HF group also had higher weight of liver than the HFI group (P=0.028). The weight of AT was significantly higher in both HF (P<0.001) and HFI (P=0.016) groups as compared with the control diet group, and was significantly lower in the HFI group as compared with the HF group (P=0.022).
Table 1. Body weight, weight of organs and F4/80 expression.
| Control diet (n=6) | High fat (n=6) | High fat+I3C (n=6) | P-valuea | |
|---|---|---|---|---|
| Body weight (g) | ||||
| Baseline | 21.7±0.5 | 21.6±1.6 | 21.6±1.8 | 0.984 |
| Week 12 | 26.6±1.0 | 34.4±2.1c | 28.3±2.0d | <0.001b |
| Organ weight (g) | ||||
| EAT | 0.50±0.11 | 1.81±0.60c | 1.18±0.15c,d | <0.001b |
| Liver | 0.95±0.10 | 1.05±0.08 | 1.10±0.07c | 0.032b |
| Heart | 0.47±0.08 | 0.42±0.08 | 0.42±0.08 | 0.454 |
| Kidney | 1.13±0.10 | 1.02±0.12 | 1.05±0.08 | 0.160 |
| Spleen | 0.28±0.08 | 0.23±0.05 | 0.25±0.05 | 0.381 |
| Relative F4/80 expression (divided by β-actin expression) | 1.0 | 10.8±2.8c | 3.2±0.8d | 0.019b |
Abbreviation: EAT, epididymal adipose tissue.
Data were presented as mean±standard deviation and tested by ANOVA. Tukey's test or Dunnett's T3 test was used for post-hoc tests.
Significantly different among three groups, P<0.05.
Significantly different from control diet group, P<0.05.
Significantly different from high fat group, P<0.05.
Effects of I3C on macrophage infiltration in AT
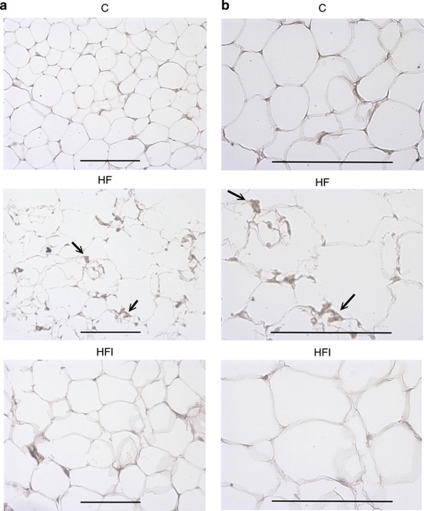
AT during obesity is characterized by increased infiltration by macrophages. After the animals were fed the different diets for 12 weeks, histological examination using anti-F4/80 antibodies revealed appreciable positive staining in epididymal AT of mice fed with HF. However, there was no significant difference in the AT between the control group and the HFI group (Figure 1 and Table 1). The macrophage-specific marker, F4/80, is expressed significantly higher in the HF group as compared with the control diet group (P=0.001), and significantly lower in the HFI group as compared with the HF group (P=0.004). The similar levels of F4/80 in the control group and HFI group indicate that I3C prevents HF-induced infiltration by macrophages.
Figure 1.
Effects of I3C on F4/80 expression in epididymal adipose tissue (AT), × 200 magnification (a) and × 400 magnification (b), in animals fed different diets for 12 weeks (C, control diet; HF, high-fat diet; HFI, high-fat diet+intraperitoneally administered I3C). Immunohistochemistry detected a macrophage-specific antigen (F4/80) in epididymal AT from high-fat diet-induced obese mice. The black arrows showed abundant macrophages within AT. Macrophages were observed both near blood vessels and among large adipocytes. The scale bar represents 100 μm.
Effects of I3C on inflammatory mediators in adipocytes co-culture with macrophages
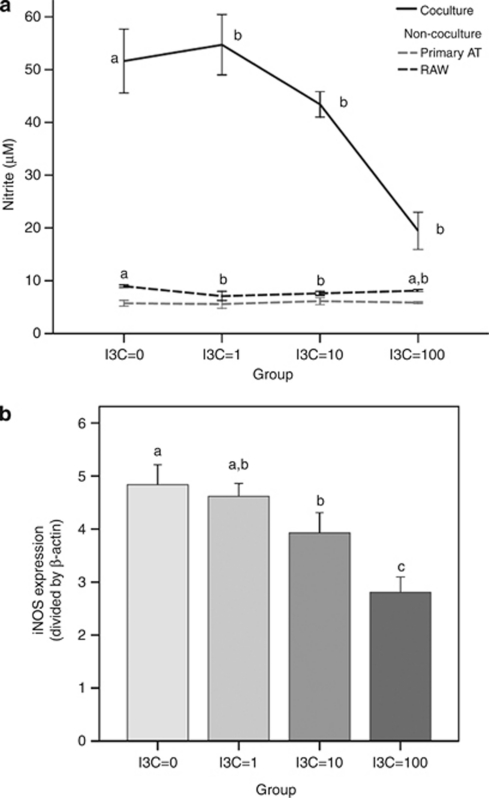
Differentiated adipocytes were co-cultured with RAW 264.7 cells for 24 h and then treated with I3C (zero to 100 μ) for 24 h. As shown in Figure 2a, the nitrite decreased as the concentration of I3C increased (P<0.001). The I3C treatment suppressed nitrite by 62.3% at 100 μ I3C, 15.9% at 10 μ, 6.0% at 1 μ compared with no I3C added. In primary adipocytes or RAW macrophages cultured alone, the concentration-response inhibitive effect of I3C on nitrite release was not evident (Figure 2a). Interestingly, the nitrite production was dramatically increased during the co-culture of adipocytes and macrophage compared with the two cells cultured alone (Figure 2a).
Figure 2.
Effects of I3C on nitrite content and inducible nitric oxide synthase (iNOS) mRNA expression in co-culture of primary adipocytes and macrophages. Differentiated adipocytes were co-cultured with RAW 264.7 macrophages (1 × 106 cells per well) or the cells were cultured alone (a) and cells were treated with I3C (μ) for 24 h. Nitrite levels were spectrophotometrically measured with Griess reagent. Expression of iNOS mRNA (b) was measured by reverse transcriptase polymerase chain reaction. Values are the mean±s.d. from six measurements. Values with different superscript letters (a, b and c) indicate significant differences between groups (P<0.05).
We also investigated the effect of I3C on expression of iNOS mRNA in co-culture of adipocytes and macrophages by reverse transcriptase polymerase chain reaction (Figure 2b). The level of iNOS mRNA expression changed downward as the concentration of I3C increased (P=0.001). The level of iNOS mRNA expression at concentration=10 μ was lower than at concentration=0 μ (P=0.038), and the level of iNOS mRNA expression at concentration=100 μ was lower than at other concentrations (P<0.01). The results indicate that the treatment of I3C reduced expression of iNOS.
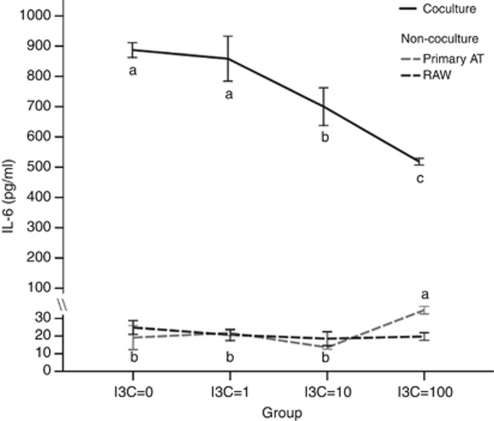
A strong effect was found from measuring IL-6 expression. High expression of IL-6 was detected when adipocytes were co-cultured with RAW 264.7 macrophages (Figure 3). Expression of IL-6 was reduced by adding I3C in a concentration-dependent manner. Added I3C suppressed the level of IL-6 by 21.1% at 10 μ I3C (P=0.008) and 41.6% at 100 μ I3C (P<0.001), respectively. However, the expression of IL-6 in primary adipocytes or RAW 264.7 macrophages was very low when cultured alone, with or without I3C treatment (Figure 3), which was less responsive to I3C.
Figure 3.
Effects of I3C on interleukin-6 (IL-6) in co-culture of primary adipocytes and macrophages. Differentiated adipocytes were co-cultured with RAW 264.7 macrophages (1 × 106 cells per well), and cells were treated with I3C (μ) for 24 h. IL-6 contents in the co-culture model, primary adipocytes alone and RAW 264.7 macrophages alone, were measured by ELISA. Values are the mean±s.d. from six measurements. Values with different superscript letters (a, b and c) indicate significant differences between groups (P<0.05).
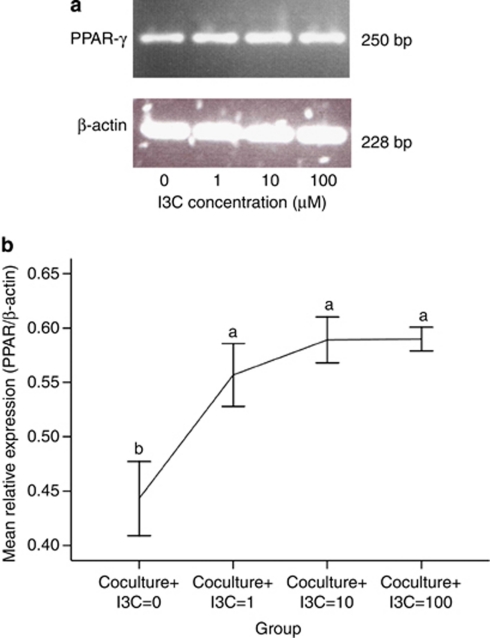
Effects of I3C on the expression of PPAR-γ mRNA
The expression of PPAR-γ was examined by reverse transcriptase polymerase chain reaction (Figure 4a). As shown in Figure 4b, I3C stimulated the relative expression of PPAR-γ mRNA in adipocytes co-cultured with macrophages (compared with I3C=0, P<0.001). Even though PPAR-γ mRNA expression increased gradually as a function of I3C concentration, differences in the level of expression were not significant in response to 1, 10 or 100 μ I3C.
Figure 4.
Effects of I3C on peroxisome proliferator-activated receptor (PPAR-γ) mRNA in co-culture of primary adipocytes and macrophages. Differentiated adipocytes were co-cultured with RAW 264.7 macrophages (1 × 106 cells per well), and cell treated with I3C for 24 h. Differentiated adipocytes were treated with ethanol (0 μ I3C), 1 μ I3C, 10 μ I3C or 100 μ I3C, as indicated. The expression of PPAR-γ mRNAs was determined by reverse transcriptase polymerase chain reaction. The total cellular RNA was isolated and reverse-transcribed to cDNA (a). The relative expression of PPAR-γ under different concentrations of I3C was summarized (b). These experiments were repeated three times. Values with different superscript letters (a and b) indicate significant differences between groups (P<0.05).
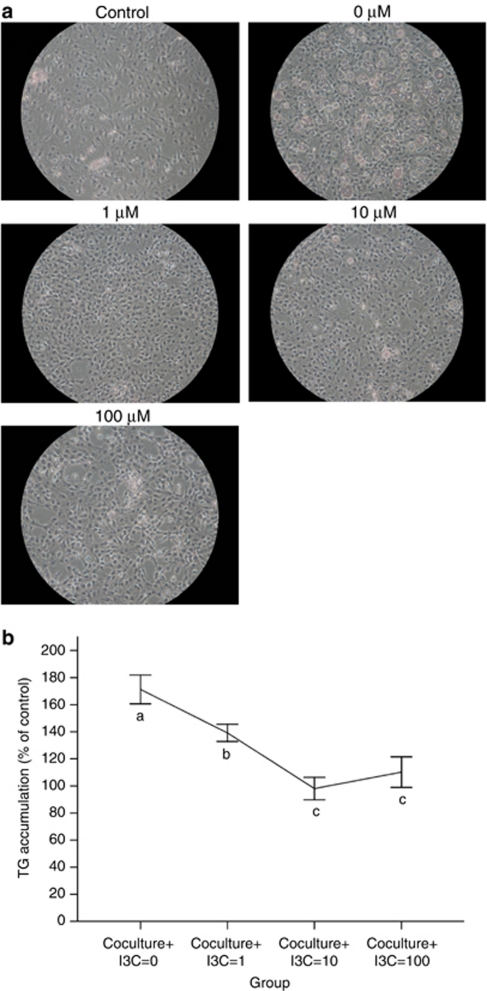
Effects of I3C on intracellular lipid accumulation
We next measured adipogenesis, and determined whether adipogenesis is affected by I3C in 3T3-L1 cells. The 3T3-L1 cell line is widely used as a model of adipocyte differentiation and adipose biology.31 After differentiation, the mature 3T3-L1 adipocytes accumulated larger intracellular droplets than the undifferentiated preadipocytes. Histological results demonstrated that I3C reduced the content of intracellular triglyceride in 3T3-L1 adipocytes (Figure 5a). The data are summarized in Figure 5b. Triglyceride accumulation was curtailed from 171.3% at concentration=0 μ, 139.2% at concentration=1 μ to 98.1% at concentration=10 μ. Although the triglyceride accumulation increased slightly between concentration=10 μ and concentration=100 μ, the change was not significant (P=0.434). I3C treatment resulted in a reduction in intracellular triglyceride accumulation, which was similar in extent to the reduction seen for inflammatory cytokines (Figure 3).
Figure 5.
Effects of I3C on intracellular lipid accumulation (a) and triglyceride accumulation (TG) content (b) in 3T3-L1 adipocytes. 3T3-L1 were differentiated by culturing with adipogenic agent. Cells were treated with various concentrations of I3C for 72 h. Cells were stained with oil red O. TG contents were quantified by a commercial assay kit, as described in Materials and Methods. These experiments were repeated three times, and similar results were obtained. Control: undifferentiated 3T3-L1 preadipocytes; 0 (0 μ I3C): differentiated 3T3-L1 adipocytes treated with ethanol; 1–100 μ: differentiated adipocytes treated with 1–100 μ I3C. Values are the mean±s.d. from three measurements. Values with different superscript letters (a, b, and c) indicate significant differences between groups (P<0.05).
Discussion
In the present study, we demonstrated that I3C reduces macrophage accumulation in obese AT and also reduces inflammatory changes, using the experimental model of co-cultured adipocytes and macrophages. Obese AT is characterized by an enhanced-macrophage infiltration. In white AT, the F4/80 marker for macrophages was predominantly expressed in the stromal vascular fraction where macrophages reside. We have found that the macrophage-specific marker, F4/80, is dramatically upregulated in white AT of obese mice, indicating an increase in macrophage infiltration in this tissue, and that administering I3C reduced macrophage infiltration. Obese AT is characterized by the infiltration of macrophages, and these macrophages are an important source of inflammation in that tissue.10 Dandona et al.35 indicated that white AT inflammation may be potential mechanism by which obesity leads to insulin resistance. Diet-induced obesity is associated with adipocyte hypertrophy and macrophage infiltration. We found that I3C can reverse the accumulation of white AT macrophages in obese AT, which contributes to the chronic inflammation response of obesity.
The co-culture system provides a unique in vitro experimental system to assess the expression of inflammatory markers, arising from interactions between adipocytes and macrophages. Additionally, an increased number of macrophages resident in human AT has been reported in obesity. This increase is expected to contribute to inflammatory processes by the macrophage's expression of pro-inflammatory cytokines such as tumor necrosis factor-α, IL-6 and MCP-1.36 In the present study, the data showed that the co-culture of hypertrophied adipocytes and RAW 264.7 macrophages stimulated the production of proinflammatory mediators, nitrite and IL-6, a finding consistent with a previous report.16 Compounds from cruciferous vegetables, such as I3C, have been demonstrated to have anti-cancer and anti-inflammation properties.37 Recently, we demonstrated that I3C suppressed lipopolysaccharide-induced iNOS, nitric oxide and proinflammatory cytokines.22 Obese subjects demonstrated higher levels of MCP-1 gene expression.38 Although MCP-1 expression from primary adipocytes co-cultured with macrophages was measured in response to I3C, the effects were marginal and failed to demonstrate a significant concentration-response effect (data not shown). Because MCP-1 is expressed by adipocytes and has an important role in monocyte recruitment, it is possible that obesity-related adipocyte expression of MCP-1 results in the recruitment of resident monocytes, which then elaborate inflammatory cytokines, resulting in insulin resistance and many of the features of metabolic syndrome. These observations suggested that I3C ameliorated in obese-induced AT inflammation by macrophages, both in vivo and in vitro.
We also found that I3C inhibited nitrite content and iNOS mRNA expression in adipocytes cultured with macrophages, revealing the inhibitory effect of I3C on nitric oxide production is associated with suppressed iNOS mRNA expression. Only the highest concentrations of I3C that were used in the present study had the effect of decreasing nitrite production. Furthermore, I3C can elevate PPAR-γ mRNA expression. PPAR-γ is involved in inflammatory response. Currently, the mechanisms of PPAR-γ have anti-inflammatory actions via interference with proinflammatory transcription factors, including NFκB and AP-1.39 Previously studies showed that NFκB is one of transcription factors that regulate the gene expression of MCP-1, IL-6, tumor necrosis factor-α and iNOS.40, 41 Furthermore, inactivation of the nuclear factor-κB pathway, which induces inflammatory mediators, has led to the protection against insulin resistance.42 These findings suggested that I3C may be used for protecting against the development of obesity-related sequelae, by reducing nitrite production, by inhibiting the expression of inflammatory cytokines by macrophages or by adipocytes and by modulating the expression of PPAR-γ.
Providing I3C to cultured cells, in vitro, inhibited adipogenesis into differentiated adipocytes. The prevalence of obesity and obesity-related disorders has led to increased focus on the influence of AT mass. The adipocyte is the major site for energy storage and accumulates triglycerides during nutritional excess. Smaller adipocytes are known to secrete lower levels of inflammatory markers than larger adipocytes.43 Moreover, inhibition of adipogenesis in 3T3-L1 adipocytes by certain phytochemicals resulted from the down-regulated expression of adipogenic transcription factors, such as PPAR-γ and C/EBPα.44, 45 However, I3C treatment reduced intracellular triglyceride accumulation and the expression of inflammatory mediators regulated by adipocyte-specific transcription factors, to a similar extent. The obesity-related symptoms were therefore improved by the I3C treatment. Whether I3C inhibits adipogenesis by disrupting differentiation, or by directly suppressing triglyceride synthesis, is not clear from our studies, as changes in 3T3-L1 adipocyte differentiation were not measured. It has been established that PPAR-γ can positively regulate adipose triglyceride lipase, thereby promoting the breakdown of triglycerides.46 In response to I3C, the lowered triglyceride accumulation is, therefore, consistent with the increase in expression observed for PPAR-γ, and suggests that I3C is inhibiting triglyceride synthesis directly.
To conclude, this study demonstrated the inhibitory effect of I3C on the expression of inflammatory cytokines, such as IL-6, by adipocytes or by macrophages, in the co-culture model, and that the inhibitory effect requires the presence of both types of cells (adipocytes and macrophages). This study also demonstrated that I3C inhibits production of inflammatory mediator, nitrite, in the co-culture model, as well as adipogenesis in cultured 3T3-L1 adipocytes. One possible mechanism mediating the anti-inflammatory effects of I3C may be inhibition of macrophage-induced inflammatory changes due to PPAR-γ activation. The taking of I3C as a dietary supplement or even as a prescribed drug in the future, may be a useful phytochemical for suppressing chronic inflammation in obese AT, and preventing obesity-related diseases.
The authors declare no conflict of interest.
References
- Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. Ludwig, a potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol. 2009;20:370–378. doi: 10.1097/MOL.0b013e3283309848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology. 2009;55:379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Wasserman F. Handbook of Physiology. American Physiology Society: Washington, DC; 1965. [Google Scholar]
- Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, et al. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol. 2009;183:5948–5956. doi: 10.4049/jimmunol.0901186. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon CJ, Evans CJ, Bill BR, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, et al. Fruit and vegetable intake and risk of cardiovascular disease: the women's health study. Am J Clin Nutr. 2000;72:922–928. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- Shertzer HG, Senft AP. The micronutrient indole-3-carbinol: implications for disease and chemoprevention. Drug Metabol Drug Interact. 2000;17:159–188. doi: 10.1515/dmdi.2000.17.1-4.159. [DOI] [PubMed] [Google Scholar]
- Rogan EG. The natural chemopreventive compound indole-3-carbinol: state of the science. In Vivo. 2006;20:221–228. [PubMed] [Google Scholar]
- Chen YH, Dai HJ, Chang HP. Suppression of inducible nitric oxide production by indole and isothiocyanate derivatives from Brassica plants in stimulated macrophages. Planta Med. 2003;69:696–700. doi: 10.1055/s-2003-42790. [DOI] [PubMed] [Google Scholar]
- Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317–32325. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- Khor TO, Keum YS, Lin W, Kim JH, Hu R, Shen G, et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006;66:613–621. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]
- Agrawal RC, Kumar S. Prevention of cyclophosphamide-induced micronucleus formation in mouse bone marrow by indole-3-carbinol. Food Chem Toxicol. 1998;36:975–977. doi: 10.1016/s0278-6915(98)00032-5. [DOI] [PubMed] [Google Scholar]
- Machijima Y, Ishikawa C, Sawada S, Okudaira T, Uchihara JN, Tanaka Y, et al. Anti-adult T-cell leukemia/lymphoma effects of indole-3-carbinol. Retrovirology. 2009;6:7. doi: 10.1186/1742-4690-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HP, Tsai IH, Huang SY, Dai PH, Chen YC, Chen YH. Study of contents of indole glucosinolates in fresh and pickled cruciferous vegetables cultivated in Taiwan. Nutr sci J. 2007;32:102–107. [Google Scholar]
- Reed GA, Peterson KS, Smith HJ, Gray JC, Sullivan DK, Mayo MS, et al. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14:1953–1960. doi: 10.1158/1055-9965.EPI-05-0121. [DOI] [PubMed] [Google Scholar]
- Wong GY, Bradlow L, Sepkovic D, Mehl S, Mailman J, Osborne MP. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J Cell Biochem Suppl. 1997;28-29:111–116. doi: 10.1002/(sici)1097-4644(1997)28/29+<111::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bradlow HL, Michnovicz J, Telang NT, Osborne MP. Effects of dietary indole-3-carbinol on estrogen metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991;12:1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974;1:113–116. [Google Scholar]
- Takahashi N, Kawada T, Yamamoto T, Goto T, Taimatsu A, Aoki N, et al. Overexpression and ribozyme-mediated targeting of transcriptional coactivators CREB-binding protein and p300 revealed their indispensable roles in adipocyte differentiation through the regulation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2002;277:16906–16912. doi: 10.1074/jbc.M200585200. [DOI] [PubMed] [Google Scholar]
- Privat C, Lantoine F, Bedioui F, Millanvoye van Brussel E, Devynck J, Devynck MA. Nitric oxide production by endothelial cells: comparison of three methods of quantification. Life Sci. 1997;61:1193–1202. doi: 10.1016/s0024-3205(97)00661-9. [DOI] [PubMed] [Google Scholar]
- Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglyceride by staining intercytoplasmic lipids with Oil Red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues—association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- Weng JR, Tsai CH, Kulp SK, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008;262:153–163. doi: 10.1016/j.canlet.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond) 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rangan GK, Goodwin B, Tay YC, Harris DC. Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-κB dependent. Kidney Int. 2000;57:2011–2022. doi: 10.1046/j.1523-1755.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Jernås M, Palming J, Sjöholm K, Jennische E, Svensson PA, Gabrielsson BG, et al. Seperation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- Lin J, Della-Fera MA, Baile CA. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes Res. 2005;13:982–990. doi: 10.1038/oby.2005.115. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, et al. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2005;338:694–699. doi: 10.1016/j.bbrc.2005.09.195. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Schupp M, Guan HP, Gardner NP, Lazar MA, Flier JS. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007;293:E1736. doi: 10.1152/ajpendo.00122.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]