Several novel strategies using engineered receptors activated by synthetic ligands or by light have recently ushered in a new era of brain research that allows for precise experimental manipulation of neuronal activity. These techniques are now being used to probe the involvement of discrete brain circuits in complex behaviors.
One such approach uses designer receptors exclusively activated by designer drugs (DREADDs) to modulate cellular functions (Rogan and Roth, 2011). This family of evolved muscarinic receptors has been shown to increase (Gs-DREADD; Gq-DREADD) or decrease (Gi/o-DREADD) cellular activity following administration of an otherwise inert synthetic ligand, clozapine-n-oxide (Armbruster et al, 2007). When packaged into viral vectors or expressed in transgenic mouse models, these tools allow cellular activity to be controlled in a defined spatial and temporal manner. For example, activation of hippocampal neurons by Gq-DREADD receptors amplifies γ-rhythms and increases locomotor activity and stereotypy in mice (Alexander et al, 2009). Activity of non-neuronal cells can also be controlled by DREADD receptors, as expression and activation of either Gs-DREADD or Gq-DREADD receptors in pancreatic β-cells increases insulin release, and repeated activation of these receptors leads to β-cell hypertrophy (Guettier et al, 2009).
We have been using DREADD receptor technology in a cell-specific manner to unravel the role of striatal circuits in neuropsychiatric disorders, such as drug addiction and obsessive–compulsive disorder. We developed viral vectors that use neuropeptide promoters (dynorphin or enkephalin) to target DREADD receptor expression to specific cell populations in the striatum (striatonigral versus striatopallidal neurons, respectively). We found that transiently decreasing activity of striatopallidal neurons in rats during repeated amphetamine exposure facilitated the development of behavioral sensitization, whereas disrupting activity of striatonigral neurons impaired the persistence of this phenomenon (Ferguson et al, 2011). Thus, these findings clearly demonstrate that striatonigral and striatopallidal neurons have critical, yet opposing, roles in the regulation of drug experience-dependent behavioral plasticity.
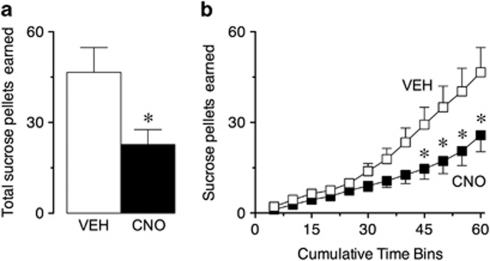
More recently, we have examined the role of striatonigral neurons in a simple operant learning task. We found that transiently inhibiting activity of these neurons in the dorsal striatum of rats interfered with the acquisition of lever pressing for a sugar reward (see Figure 1), indicating that activation of the striatonigral pathway is required for action–outcome learning. This data suggests that dysregulation of striatal circuits may therefore contribute to the development of the aberrant reward and reinforcement learning that is common to many neuropsychiatric disorders.
Figure 1.
Transiently reducing excitability of striatonigral neurons impairs action–outcome learning of a simple operant task. (a) Activation of Gi/o-designer receptors exclusively activated by designer drug (DREADD) receptors in striatonigral neurons in the dorsal striatum by clozapine-n-oxide (CNO) significantly decreased the total number of sucrose pellets that rats earned during a 1-h operant test session, compared with vehicle (VEH)-treated rats. t-test, T17=2.65, *P=0.02 (b) Significant differences in the number of sucrose pellets earned between groups were seen in the last quarter of the session (45–60 min time points), suggesting an impairment in learning acquisition in the CNO-treated rats. Two-way repeated measures analysis of variance (ANOVA), main effect of time: F1,17=41.74, P<0.0001; main effect of pretreatment: F1,17=6,98, P=0.017; interaction between time and pretreatment factors: F1,17=6.57, P<0.0001; *P<0.05, Bonferroni's post-hoc test. Data represent mean SEM. N=7–12/group.
Increasingly selective pharmacological ligands have not necessarily improved the efficacy of drug treatments for neuropsychiatric disorders. Although safe and effective viral vectors for delivery of engineered receptors into humans still need to be refined, these tools may provide a distinct alternative to the currently available brain stimulation methods that are being used in the treatment of neuropsychiatric disorders. Thus, in addition to allowing us to parse the contribution of specific neural circuits in behaviors, perhaps engineered receptors may one day also offer the opportunity to provide therapeutic value without the ‘dread'-ed side effects.
The authors declare no conflict of interest.
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]