Autism is a widespread disorder characterized by deficits in social interactions, impairments in communication, and repetitive and stereotypic behaviors. Identification of genetic markers has proved difficult, owing to the highly complex and variable nature of the disease. Although autism spectrum disorders (ASDs) arise as a consequence of mutations in genes with multiple molecular functions, they appear to converge on common biological pathways to give rise to autism-relevant behaviors (Abrahams and Geschwind, 2008). One such pathway is the PI3K-mammalian target of rapamycin (mTOR) signaling cascade. The mTOR pathway is a central regulator of cell growth, proliferation, survival, and cap-dependent protein translation. In brain, components of the mTOR pathway are present at synapses, where they regulate dendritic spine morphology, and are essential to synaptogenesis. Growing evidence indicates that dysregulation of mTOR is involved in human diseases, including cancer, diabetes, and autism. Mutations in tuberous sclerosis complex (TSC)1, TSC2, neurofibromatosis 1 (NF1), and PTEN lead to overactivated PI3K-mTOR pathway, autism-relevant behaviors, and tuberous sclerosis, neurofibromatosis, or macrocephaly.
Fragile X syndrome (FXS) is the most common heritable form of intellectual disabilities, and a leading genetic cause of autism. Recent findings that PI3K-mTOR signaling is overactivated at synapses of Fragile X mice (Sharma et al, 2010) and in humans with FXS provide the first evidence that genetic mutation not only of components within the mTOR signaling cascade, but also distant regulatory proteins, can lead to autism-related phenotypes. Overactivated mTOR signaling is linked to elevated cap-dependent translation and impaired synaptic plasticity in Fragile X mice (Sharma et al, 2010). mGluR1/5 links via Homer to PIKE (PI3 kinase enhancer) at synapses, where it engages PI3K–mTOR signaling in response to synaptic stimulation. PIKE, an upstream activator of mTOR and identified target of FMRP (Darnell et al, 2011) is elevated at the synapses of Fmr1 KO mice (Gross et al, 2010; Sharma et al, 2010), providing a functional link between loss of FMRP and overactivated mTOR signaling (Figure 1). These findings identify dysregulation of mTOR signaling as a phenotypic feature common to FXS, TSC1 and 2, NF1, and PTEN-associated autism syndromes. Whereas other syndromic ASDs arise from mutations in components of the PI3K–mTOR pathway, FXS arises from silencing of the gene encoding FMRP, an RNA-binding protein that represses translation of a large array of RNAs including PIKE. This, in turn, results in elevation of PIKE and overactivation of PI3K–mTOR signaling. These observations raise the possibility that dysregulation of mTOR may be a unifying theme in a growing number of ASDs and ASD-associated syndromes.
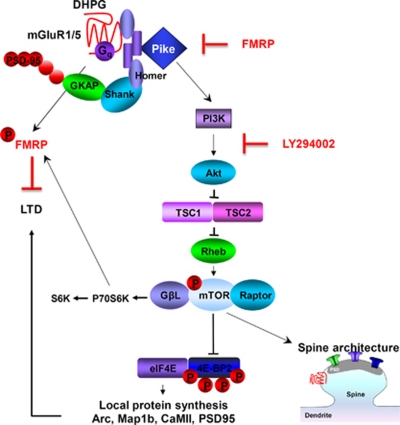
Figure 1.
Scheme showing the link between deficits in FMRP and overactivated mTOR. Our findings support a model where, in WT mice, FMRP represses PIKE, an upstream activator of PI3K signaling and identified target of FMRP, and thereby inhibits mTOR signaling. Upon activation, group I mGluRs act via Gq and Homer to bind PIKE and engage PI3K signaling, which activates mTOR. mTOR drives cap-dependent translation and local synthesis of synaptic proteins such as Arc, Map1b, CaMII, and PSD-95, critical to mGluR-LTD. In addition, mTOR regulates LIMK and cofilin, which promote spine morphogenesis. In mice lacking FMRP, PIKE is derepressed, resulting in overactivation of mTOR, accumulation of synaptic proteins, and exaggerated, protein synthesis-independent LTD. The PI3K inhibitor LY294002 corrects p-mTOR and restores DHPG sensitivity. A prediction of the model is that dysregulation of mTOR signaling contributes to the cognitive and social interaction deficits observed in humans with Fragile X.
On the basis of the clear link between overactivated mTOR signaling and autism, the mTOR pathway represents a promising therapeutic target for the treatment of ASDs. Treatment with the mTORC1 inhibitor rapamycin has shown promising results in PTEN knockout mice (Zhou et al, 2009) and TSC2+/− mice (Ehninger et al, 2008). Thus, interventions that target mTOR signaling should be at the leading edge of future translational research in the autism field.
Acknowledgments
This work was supported by generous grants from the Fragile X Syndrome Research Foundation and the National Institutes of Health Grants MH092877. RSZ is the FM Kirby Professor in Neural Repair and Protection.
The authors declare no conflict of interest.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE.et al (2011FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism Cell 146247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]