Abstract
A 68-year-old man presented with anorexia, weight loss and severe asthenia starting 2 months before and fever lasting over 2 weeks. Analytical study revealed increased C reactive protein and sedimentation velocity. While thoracic x-ray and urinalysis were unremarkable, blood cultures were consistently positive for Enterococcus faecalis. A thoracic echocardiogram revealed a very large vegetation adherent to the ventricular electrocatheter of the patient’s pacemaker, so parenteral antibiotics were started. During his stay at the internal medicine ward, patient suffered three syncopal episodes and maintained intermittent fever. A transesophageal ECG performed at a tertiary centre on the 14th day unmasked a 9–12 cm2 organised mass, ovoid and with regular borders, adherent to the ventricular electrocatheter at right atrium level and protruding to the ventricle at systole. Patient was referred for cardiothoracic surgery and a 13 cm2 mass was removed alongside the ventricular electrocatheter. Two weeks following surgery, patient remains stable and asymptomatic.
Background
This is an unusual presentation of device related infective endocarditis, a severe disease associated with high mortality risk. Syncope is not a usual manifestation of infectious endocarditis (especially in right sided infective endocarditis) and, although vegetations may grow to significant dimensions, it is extremely rare to find such voluminous intracardiac masses in patients with this condition.
Case presentation
A 68-year-old man presented to the emergency ward of a District Hospital with a progressively aggravating clinical condition subsisting for 2 months and consisting of anorexia, weight loss and severe asthenia. Patient also mentioned refractory fever in the 2 weeks before admission. His past medical history included diabetes mellitus type 2, ischemic cardiomyopathy with moderate to severe left ventricle dysfunction (documented at a routine echocardiogram performed nine months before) and a three day stay at the cardiology department, in 2002, for elective dual chamber pacemaker implantation for intermittent complete atrioventricular block (with subsequent generator replacement in 2008). Chronic medication included oral antidiabetics, aspirin, simvastatin and perindopril. Remaining medical history was unremarkable, including the absence of previous syncopal episodes. Physical examination at admission revealed mild pallor and dehydration, hyperthermia (38.3°C) and a I-II/VI holosystolic murmur best audible at the lower left sternal border. No adventitia or respiratory distress was present, pacemaker function was appropriate and remaining examination was negative for any significant findings.
Investigations
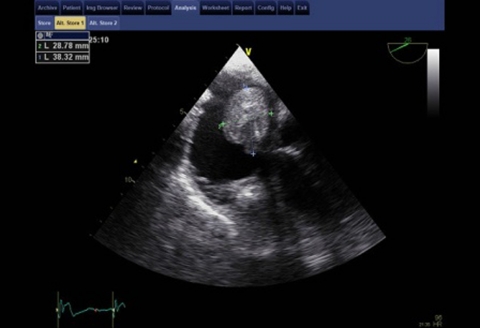
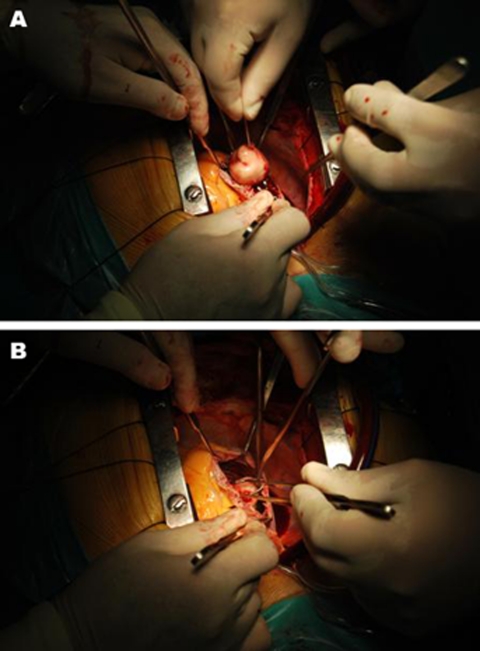
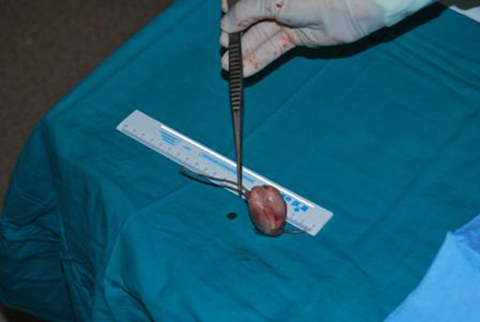
Biochemical and haematological investigations revealed elevated C reactive protein (16.5 mg/dl) and sedimentation velocity (82/1°h), normochromic normocytic anemia (haemoglobin 9.5 g/dl) and creatinine levels estimating creatinine clearance at 78 ml/min. Thoracic chest scan and urinalysis were normal. Blood cultures taken at admission (three samples) and repeated later on were consistently positive for E faecalis. Admission transthoracic echocardiogram showed a very large intracardiac mass adherent to the ventricular electrocatheter at right atrium level, which led to beginning of parenteral antibiotic therapy for device related infective endocarditis. Following repetitive syncopal episodes, the recurrence of fever after some days of apyrexia and new signs of right heart failure (namely hepatomegaly with abnormalities in liver function tests and mild-moderate peripheral oedema), the echocardiogram was repeated on the 12th day, revealing an apparent moderate increase in the size of the suspected vegetation. Patient was sent to a tertiary centre for transesophageal echocardiography and observation by the arrhythmology department. This last exam (performed on the 14th day) unmasked an apparently 9–12 cm2 sized organised mass, ovoid and with regular borders, adherent to the ventricular electrocatheter at right atrium level and clearly protruding to the ventricle at each systole (figure 1; videos 1–4). There were no signs of direct aortic, mitral or tricuspid involvement. The systolic protrusion of the vegetation to the right ventricle inflow tract was the probable cause of the repetitive syncopes. These findings led to case discussion at the cardiothoracic surgery department and ultimately patient was referred for surgical mass and lead extraction, exposing a 13 cm2 infected mass adherent to the electrocatheter (figures 2 and 3).
Figure 1.
Transesophageal echocardiogram showing a 28.78×38.32 mm vegetation adherent to the pacemaker lead at 25° 2D view.
Figure 2.
(A, B) In vivo pictures of the infected intracardiac mass during surgical extraction.
Figure 3.
Mass size after excision.
Video 1.
The video clips show the vegetation in its original position and protruding to the right ventricle: 4-chamber apical view, transthoracic echocardiogram.
Video 2.
The video clips show the vegetation in its original position and protruding to the right ventricle: transesophageal echocardiogram at 0°.
Video 3.
The video clips show the vegetation in its original position and protruding to the right ventricle: 90°.
Video 4.
The video clips show the vegetation in its original position and protruding to the right ventricle: 26°.
Differential diagnosis
The constitutional symptoms and analytical results at admission suggested an infectious disease, even though neoplastic and auto-immune processes could not be excluded. The latter seemed unlikely though, given the usual earlier presentation for the most common auto-immune diseases. Neoplasms are the second most common cause of unexplained fever (mainly lymphoma and haematological malignancies, colon and renal cell carcinoma and liver involvement). According to the modified duke criteria,1 the findings of consistently positive blood cultures for a typical microorganism consistent with infective endocarditis and an intracardiac mass in a patient with a predisposing heart condition led to an early diagnosis of device related infective endocarditis. Nevertheless, some of the characteristics of the intracardiac mass, namely its regular borders and ovoid outlook (vegetations are usually irregularly shaped and exhibit chaotic motion), could not rule out the possibility of a secondarily infected catheter-adherent thrombus or non-bacterial thrombotic endocarditis (marantic endocarditis). Formation of thrombus in the right side of the heart is rare in the absence of right ventricular dysfunction/dilatation, yet a venous thrombus could have embolised and become entrapped on the pacer wires or a thrombus could have been primarily formed on the electrocatheter and later become infected in the context of a bacteraemia. This would have impacted on subsequent therapeutic decisions, as antithrombotic therapy would be warranted in case of a thrombus while there is no indication for the initiation of antithrombotic drugs during the active phase of infective endocarditis due to the high risk of intracranial haemorrhage (especially in those with Streptococcus aureus prosthetic valve endocarditis or with previous neurological events, which was not the case).2 As the patient had neither right ventricular dysfunction/dilation nor signs or history of deep vein thrombosis, the intracardiac mass was assumed as a vegetation from infective endocarditis.
Treatment
Patient was initially started on intravenous ceftriaxone 1g daily and vancomycin 1 g every12 h (with subsequent adjustments according to drug levels). On the 7th day, bearing in mind the persistency of fever, ceftriaxone was substituted for gentamicin 80 mg every 8 h (also, with adjustments of dosage according to drug levels). Following the aggravation of the clinical status, the performance of new echocardiographic studies (transthoracic and trans-oesophageal) and the discussion of the case in the arrhythmology department of a tertiary centre, patient was referred for a cardiothoracic surgery centre for urgent surgical excision of the intracardiac mass. Indications for this decision lay on the persistency of fever (sign of uncontrolled infection and risk factor for complicated course) and the surprisingly large vegetation size (roughly 4.5×3 cm).3 4 Also, in most patients with device related infective endocarditis, prolonged antibiotic therapy must be associated with device removal.5 Percutaneous extraction is recommended in most patients, including those with larger vegetations (> 10 mm).6 However, surgical extraction should be considered in case of very large (> 25 mm) vegetations or when percutaneous removal seems technically impossible.7 Surgery required good exposure under extracorporeal circulation, as recommended, to enable the complete removal of all infected material. Reimplantation of a new device was postponed considering the risk of reinfection and the fact that patient remained stable, asymptomatic and with no ECG documentation of atrioventricular block despite continuous rhythm monitoring.
Outcome and follow-up
Two weeks following surgery, patient remains haemodynamically stable, apyretic, asymptomatic, with no signs of right sided heart failure. He remains in sinus rhythm with first degree atrioventricular block. No syncopal or presyncopal episodes have been reported. A new pacing system will be implanted on the contralateral side as soon as patient finishes his 6-week antibiotic cycle.
Discussion
Intravascular devices such as pacemakers, implantable cardioverter-defribillators, left ventricular assist devices and prosthetic vascular grafts are life saving therapies for patients with malignant arrhythmias, heart failure or severe valvular diseases. As indications for their use have increased, so has the prevalence of infectious or thrombotic complications associated with those devices. We present the case of a patient with a dual chamber pacemaker implanted some years before who is admitted for a condition suggestive of either device related infective endocarditis or infected thrombus.
Multiple reports of device related infective endocarditis have been published,8 9 including some with large (> 10 mm) associated vegetations.10 11 Nevertheless, to our knowledge, never has such large (roughly 45×30 mm) sized vegetation been mentioned in any report, which raises some questions regarding the best therapeutic options. Calton R et al reported an infected implantable cardioverter-defibrillator (ICD) lead with a 41×12.5 mm vegetation successfully removed by laser extraction as patient’s general poor condition disqualified him for cardiosurgical intervention with the use of cardiopulmonary bypass.12 Antibiotic therapy is certainly warranted, yet the optimal method and timing for mass extraction are controversial. Some studies designed to evaluate the outcome of an approach combining antibiotic therapy with non-surgical transvenous complete removal for the management of cardiac device infections concluded that most cases can be treated with antibiotic therapy and non-surgical removal of the entire infected device, thus allowing a successful reimplantation and preventing recurrent infection and operative mortality.13 This applied even to patients with larger (> 10 mm) vegetations,7 as embolism to the lung most often proceeds without haemodynamic compromise. However, no guidelines or published studies feature the proper way to deal with extremely large device adherent vegetations such as the one in our patient. The potential complications of such finding included sudden cardiac death caused by right ventricle inflow tract obstruction or massive pulmonary embolism obstructing the main pulmonary artery, right sided heart failure caused by severe right sided valvular obstruction, with secondary hepatosplenic dysfunction, pulmonary septic emboli possibly complicated by pulmonary infarction, abcess, pneumothorax and purulent pulmonary effusion.14
Percutaneous extraction of a lead with such voluminous adherent mass could lead to its embolisation and consequent obstruction of the right ventricle inflow tract, its outflow tract or even a main stem of the pulmonary artery, with likely catastrophic consequences, even though there are some very rare reports of percutaneous extraction of large vegetations using a filter in the outlet of the right ventricle, which allows for grasping the vegetation in case it is torn off as a whole.15 It neither interferes with nor protects against flowing of small fragments though. Surgical extraction was then warranted, although the 2009 European Society of Cardiology guidelines on the prevention, diagnosis and treatment of infective endocarditis considers surgical extraction for patients with very large (> 25 mm) vegetations a class IIb indication only, with a C level of evidence.16
This report has several points of interest. First, it reinforces the need for clinical suspicion of infective endocarditis in all patients with intracardiac devices and unexplained fever. Also, it shows that repetitive syncopal episodes, considerably rare in right sided infective endocarditis, may represent either a sign of right ventricle inflow or outflow tract obstruction. It unveils the possibility of development of extremely large vegetations adherent to pacemaker of defibrillator leads even before the occurrence of serious complications. Furthermore, it demonstrates that surgical extraction of infected leads is sometimes the only option despite the significant surgical mortality risk of these patients. Finally, it tells us that a happy ending is definitely possible even in the presence of multiple features that usually associate with a poor prognosis, namely a device related intracardiac infection, very large vegetation size and occurrence of heart failure. The possibility of relapse or reinfection must still be taken into account during prognostic assessment.
Learning points.
-
▶
During its course, right sided or device related infective endocarditis may present with misleading symptoms such as repetitive syncopal episodes.
-
▶
Besides antibiotic therapy (4–6 weeks in most cases), system removal is mandatory in device related infective endocarditis.
-
▶
In device related infective endocarditis, vegetations may reach extremely large sizes (> 10 cm2), warranting surgical extraction of the system and prohibiting percutaneous system removal.
-
▶
Vegetations may become extremely voluminous before any complication arises.
Acknowledgments
The authors would like to thank Dr Pedro Gomes, Dr Luísa Loureiro, Dr Teresa Alfaiate, Dr Luís Semedo and Dr António Leitão-Marques for their contributions to the patient’s care.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8 [DOI] [PubMed] [Google Scholar]
- 2.Tornos P, Almirante B, Mirabet S, et al. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 1999;159:473–5 [DOI] [PubMed] [Google Scholar]
- 3.Delahaye F, Célard M, Roth O, et al. Indications and optimal timing for surgery in infective endocarditis. Heart 2004;90:618–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksoy O, Sexton DJ, Wang A, et al. Early surgery in patients with infective endocarditis: a propensity score analysis. Clin Infect Dis 2007;44:364–72 [DOI] [PubMed] [Google Scholar]
- 5.Sohail MR, Uslan DZ, Khan AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc 2008;83:46–53 [DOI] [PubMed] [Google Scholar]
- 6.Meier-Ewert HK, Gray ME, John RM. Endocardial pacemaker or defibrillator leads with infected vegetations: a single-center experience and consequences of transvenous extraction. Am Heart J 2003;146:339–44 [DOI] [PubMed] [Google Scholar]
- 7.Ruttmann E, Hangler HB, Kilo J, et al. Transvenous pacemaker lead removal is safe and effective even in large vegetations: an analysis of 53 cases of pacemaker lead endocarditis. Pacing Clin Electrophysiol 2006;29:231–6 [DOI] [PubMed] [Google Scholar]
- 8.Bulfoni A. [Bacterial endocarditis from the stimulatory unit of a cardiac pacemaker]. Clin Ter 2005;156:11–2 [PubMed] [Google Scholar]
- 9.Badra Verdú MG, Contreras AE, Bagur RH, et al. Endocarditis of pacemaker or implantable cardioverter-defibrillator leads. Rev Fac Cien Med Univ Nac Cordoba 2007;64:45–7 [PubMed] [Google Scholar]
- 10.Vaccarino GN, Nacinovich F, Piccinini F, et al. Pacemaker endocarditis: approach for lead extraction in endocarditis with large vegetations. Rev Bras Cir Cardiovasc 2009;24:570–3 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen KT, Neese P, Kessler DJ. Successful laser-assisted percutaneous extraction of four pacemaker leads associated with large vegetations. Pacing Clin Electrophysiol 2000;23:1260–2 [DOI] [PubMed] [Google Scholar]
- 12.Calton R, Cameron D, Cusimano RJ, et al. Successful laser-assisted removal of an infected ICD lead with a large vegetation. Pacing Clin Electrophysiol 2006;29:910–3 [DOI] [PubMed] [Google Scholar]
- 13.Tascini C, Bongiorni MG, Gemignani G, et al. Management of cardiac device infections: A retrospective survey of a non-surgical approach combining antibiotic therapy with transvenous removal. J Chemother 2006;18:157–63 [DOI] [PubMed] [Google Scholar]
- 14.Miró JM, del Río A, Mestres CA. Infective endocarditis and cardiac surgery in intravenous drug abusers and HIV-1 infected patients. Cardiol Clin 2003;21:167–84, v–vi [DOI] [PubMed] [Google Scholar]
- 15.Małecka B, Kutarski A, Tomaszewski A, et al. Transvenous removal of endocardial leads with coexisting great vegetation (3.5 cm)–case report. Europace 2010;12:445–6 [DOI] [PubMed] [Google Scholar]
- 16.Horstkotte D, Follath F, Gutschik E, et al. Guidelines on prevention, diagnosis and treatment of infective endocarditis: executive summary. The task force on infective endocarditis of the european society of cardiology. Eur Heart J 2009;25:267–76 [DOI] [PubMed] [Google Scholar]