Abstract
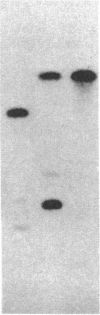
A Drosophila genomic fragment has been isolated with a deduced amino acid sequence that is strikingly homologous to that of the kinase domain of the human insulin receptor. The Drosophila DNA hybridizes with an 11-kilobase mRNA that is most prominent in 8- to 12-hr embryos. An anti-peptide antibody prepared to a sequence in the human insulin receptor kinase domain that is conserved in the Drosophila sequence immunoprecipitates a single 95-kDa Drosophila protein whose phosphorylation on tyrosine residues is dependent on insulin. We conclude that the DNA sequence is that of the kinase domain of the Drosophila insulin receptor and that the 95-kDa phosphoprotein is the autophosphorylated beta subunit of that receptor. The results are compatible with our previous reports demonstrating a specific insulin-binding Drosophila glycoprotein and an insulin-dependent tyrosine protein kinase whose activity is greatest during embryogenesis. The observations suggest a role for insulin-dependent protein tyrosine phosphorylation during embryogenesis.
Full text
PDF
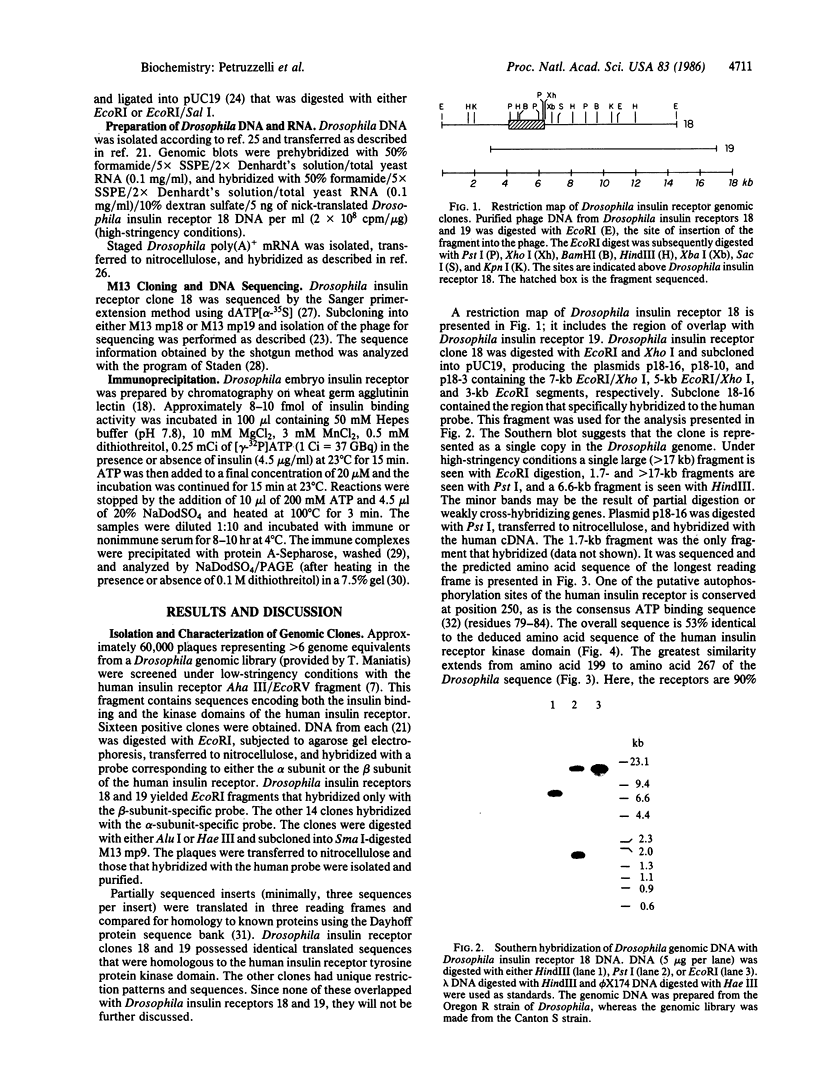



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duve H., Thorpe A. Immunofluorescent localization of insulin-like material in the median neurosecretory cells of the blowfly, Calliphora vomitoria (Diptera). Cell Tissue Res. 1979 Aug;200(2):187–191. doi: 10.1007/BF00236411. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Foster J. L., Guttman J. J., Hall L. M., Rosen O. M. Drosophila cAMP-dependent protein kinase. J Biol Chem. 1984 Nov 10;259(21):13049–13055. [PubMed] [Google Scholar]
- Herrera R., Petruzzelli L., Thomas N., Bramson H. N., Kaiser E. T., Rosen O. M. An antipeptide antibody that specifically inhibits insulin receptor autophosphorylation and protein kinase activity. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7899–7903. doi: 10.1073/pnas.82.23.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F. M., Fresco L. D., Hoffman-Falk H., Shilo B. Z. Nucleotide sequences of the Drosophila src and abl homologs: conservation and variability in the src family oncogenes. Cell. 1983 Dec;35(2 Pt 1):393–401. doi: 10.1016/0092-8674(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., Kahn C. R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J., Young M. W. The Notch locus of Drosophila melanogaster. Cell. 1983 Sep;34(2):421–433. doi: 10.1016/0092-8674(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeRoith D., Lesniak M. A., Roth J. Insulin in insects and annelids. Diabetes. 1981 Jan;30(1):70–76. doi: 10.2337/diab.30.1.70. [DOI] [PubMed] [Google Scholar]
- Livneh E., Glazer L., Segal D., Schlessinger J., Shilo B. Z. The Drosophila EGF receptor gene homolog: conservation of both hormone binding and kinase domains. Cell. 1985 Mar;40(3):599–607. doi: 10.1016/0092-8674(85)90208-9. [DOI] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. A unique proteolytic cleavage site on the beta subunit of the insulin receptor. J Biol Chem. 1981 Apr 10;256(7):3182–3190. [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Guild G. M., Prestidge L. S., Hogness D. S. A new high-capacity cosmid vector and its use. Gene. 1980 Nov;11(3-4):271–282. doi: 10.1016/0378-1119(80)90067-0. [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., Shibuya M., Hsu M. T., Wang L. H. Proto-oncogene c-ros codes for a molecule with structural features common to those of growth factor receptors and displays tissue specific and developmentally regulated expression. Mol Cell Biol. 1986 May;6(5):1478–1486. doi: 10.1128/mcb.6.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Nucleotide sequence of avian sarcoma virus UR2 and comparison of its transforming gene with other members of the tyrosine protein kinase oncogene family. J Virol. 1985 Mar;53(3):879–884. doi: 10.1128/jvi.53.3.879-884.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Garcia-Arenas R., Rosen O. M. Acquisition of insulin-dependent protein tyrosine kinase activity during Drosophila embryogenesis. J Biol Chem. 1985 Dec 25;260(30):16072–16075. [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Siegel T. W., Ganguly S., Jacobs S., Rosen O. M., Rubin C. S. Purification and properties of the human placental insulin receptor. J Biol Chem. 1981 Sep 10;256(17):9266–9273. [PubMed] [Google Scholar]
- Simon M. A., Kornberg T. B., Bishop J. M. Three loci related to the src oncogene and tyrosine-specific protein kinase activity in Drosophila. Nature. 1983 Apr 28;302(5911):837–839. doi: 10.1038/302837a0. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager H. S., Markese J., Kramer K. J., Speirs R. D., Childs C. N. Glucagon-like and insulin-like hormones of the insect neurosecretory system. Biochem J. 1976 Jun 15;156(3):515–520. doi: 10.1042/bj1560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Wadsworth S. C., Vincent W. S., 3rd, Bilodeau-Wentworth D. A Drosophila genomic sequence with homology to human epidermal growth factor receptor. Nature. 1985 Mar 14;314(6007):178–180. doi: 10.1038/314178a0. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]