Abstract
Pancreatic malignancies account for 3% of all cancer diagnoses in the UK and prognosis is poor with overall 1-year survival rates at 20% and 5-year survival rates at 5%. The majority of these cancers (75%–95%) arise from the exocrine part of the gland and are almost all invasive ductal adenocarcinomas. One per cent of all pancreatic tumours are endocrine tumours. There is limited data regarding the management of such rare neoplasms of the pancreas and some evidence suggests that prognoses and risk factors may be different. Therefore, it is important to report experience of this type of malignancy in order to build a knowledge base to guide the practice of future clinicians. The authors report a case of an intraductal tubulopapillary neoplasm of the pancreas. This is very unusual form of intraductal pancreatic tumour, which is now thought to occupy a distinct histological subcategory and has arisen within a previously irradiated field.
Background
Pancreatic malignancies account for 3% of all cancer diagnoses in the UK and the prognosis is poor with overall 1-year survival rates at 20% and 5-year survival rates at 5%.1 The majority of these cancers (75%–95%) arise from the exocrine part of the gland and are almost all invasive ductal adenocarcinomas. Other forms of exocrine neoplasia are rare and include pancreatic intraepithelial neoplasia, primary intraductal neoplasms, acinar cell adenocarcinomas, adenosquamous carcinomas, small cell carcinomas, signet ring carcinomas, hepatoid carcinomas, colloid carcinomas and undifferentiated carcinomas (with or without osteoclast like giant cells). One per cent of all pancreatic tumours are endocrine tumours.2 There are limited data regarding the management of such rare neoplasms of the pancreas and some evidence suggests that prognoses and risk factors may be different. A study undertaken at the Mayo Clinic looked at 66 patients with very rare exocrine tumours and compared them with matched controls (patients with invasive adenocarcinomas). Although no statistical difference was seen there was a clinically improved overall survival in the rare tumour group.3 Therefore, it is important to report experience of this type of malignancy in order to build a knowledge base to guide the practice of future clinicians.
We report this case of an intraductal tubulopapillary neoplasm of the pancreas to both highlight the necessity for accurate diagnosis and long-term follow-up as well as document this unusual and rare occurrence. This is very unusual form of intraductal pancreatic tumour, which is now thought to occupy its own distinct histological subcategory by demonstrating both tubulopapillary and microcystic features. Furthermore this has developed following radiation treatment for Hodgkins lymphoma and is another reported rare case of a pancreatic secondary malignancy in this setting.
Case presentation
A 50-year-old man presented in August 2008 with epigastric pain, cholestatic jaundice and anaemia. Imaging demonstrated a distal common bile duct stricture and endoscopic examination revealed an ampullary ulcer in the second part of the duodenum, the cause for which was felt to be an underlying malignancy. Although the initial biopsies were suggestive of a neuroendocrine tumour, repeat multiple endoscopic biopsies were more consistent with an adenocarcinoma. As such, the patient underwent a pylorus-preserving pancreaticoduodenectomy and cholecystectomy in October the same year. The histology obtained from the surgical specimen showed a T2 N1 low-grade undifferentiated carcinoma with angioinvasion and 1/16 lymph nodes involved. Despite multiple opinions a more detailed conclusion could not be reached regarding its origin but the consensus was a cancer of probable pancreatic origin. The patient made an uneventful postoperative recovery and was given 4 months of adjuvant gemcitabine chemotherapy as per the standard guidelines for pancreatic cancer.
The patient remained on close surveillance until February 2010 when a follow-up scan unfortunately revealed the presence of liver and nodal metastases which were felt to be unresectable. He was subsequently treated with second line combination 5-fluorouracil/oxaliplatin (FOLFOX) chemotherapy with integrated Yttrium-90 radioembolisation for downstaging. 1.5GBq Yttrium micro-spheres were delivered via the hepatic artery and following 10 cycles of FOLFOX imaging showed a partial response with reduction in both number and size of hepatic metastases. He remains on surveillance and his most recent CT scan in December 2010 showed a continued, excellent response to treatment which if maintained may render the liver lesions operable or amenable to radio-frequency ablation. If not, however, all further treatment options will be palliative.
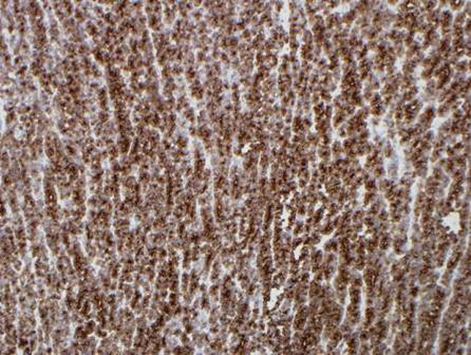
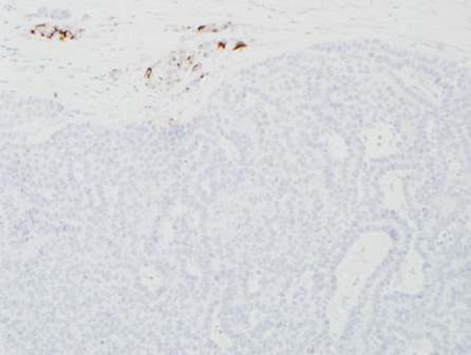
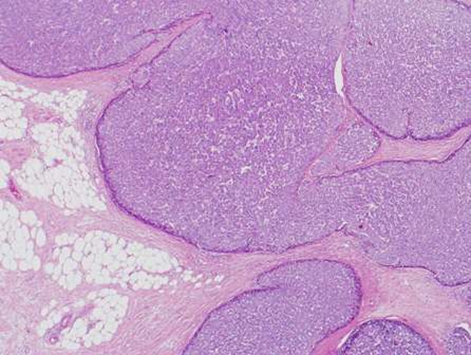
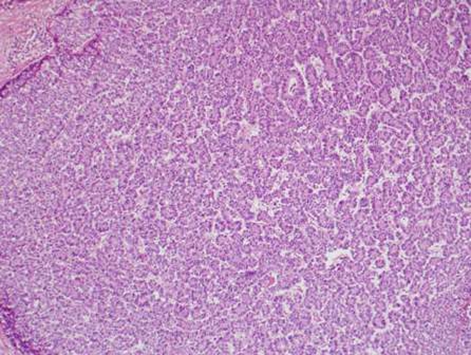
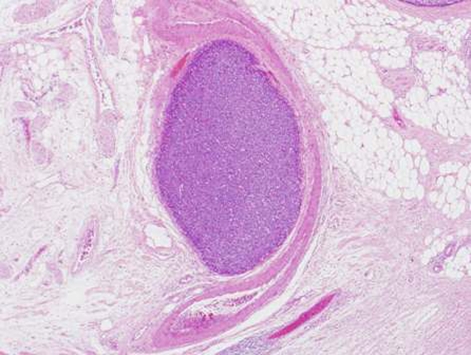
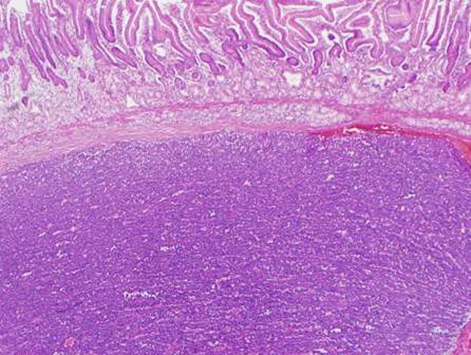
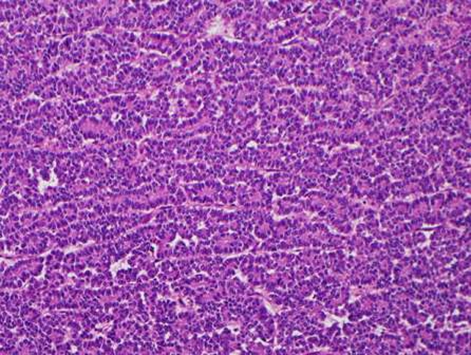
During this period, the original histology was continually re-reviewed and has finally been reported as having an intraductal tubulopapillary component and striking cytoarchitecure reminiscent of a neuroendocrine tumour, although characteristic markers such as chromogranin, CD56 and synaptophysin are negative. However cytokeratins CK7, CK18, CK19 and the mucin glycoprotein MUC6 are all strongly expressed but MUC2, MUC65A, MUC5 and p53 are negatively expressed on immunohistochemical staining. These findings, along with the malignant behaviour of the tumour and angioinvasion, are consistent with a diagnosis of an intraductal tubulopapillary neoplasm of the pancreas. Figures 1–7 are images taken which demonstrate this unique histological picture.
Figure 1.
Histology 1.
Figure 7.
Histology 7.
Figure 2.
Histology 2.
Figure 3.
Histology 3.
Figure 4.
Histology 4.
Figure 5.
Histology 5.
Figure 6.
Histology 6.
There is also a confounding medical history with the diagnosis of Hodgkins lymphoma (Ann Arbor Stage IIIB) in 1981 which was treated with laparatomy, splenectomy and para-aortic lymphadenectomy followed by adjuvant chemotherapy and radical radiotherapy. He received four cycles of the mustargen (nitrogen mustard), oncovin (vincristine), procarbazine, prednisolone (MOPP) chemotherapy regimen (nitrogen mustard, vincristine, procarbazine and prednisone) followed by 35Gy of radiation to a mantle field (upper hemibody) over 4 weeks at 1.75Gy/fraction using 4MV photons. On review of the treatment plan from the time, the epigastrium/pancreas was beneath the inferior edge of the external beam radiation field. As the current pathology in question has arisen within this previously irradiated field it is highly likely that this rare neoplasm is a secondary radiotherapy induced malignancy.
Investigations
-
▶
Histopathology – as above
-
▶
Immunohistochemistry – as above.
Differential diagnosis
Adenocarcinoma of the pancreas.
Treatment
-
▶
Pylorus-preserving pancreaticodudenectomy
-
▶
Adjuvant gemcitabine chemotherapy
-
▶
FOLFOX chemotherapy
-
▶
Integrated Ytrrium-90 microsphere radioembolisation
Outcome and follow-up
Alive.
Discussion
There have been few reported cases of intraductal tubulopapillary neoplasms of the pancreas in the literature. Although intraductal neoplasms of the pancreas are relatively uncommon they have generally been thought to fall into three main categories; pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous cystic neoplasm and intraductal tubular neoplasm.4–6 Solid pesudopapillary neoplasms of the pancreas, which occur predominantly in young women have papillary features but are regarded as a separate diagnostic entity.7 Here we describe an intraductal tubulopapillary neoplasm of the pancreas (ITPN) and the earliest reported such case exhibiting both tubulopapillary and mucinous microcystic features was in 2004 by Esposito et al. An unusual cystic, mass of the head of the pancreas was seen in a 53-year-old woman with histological analysis and immunohistochemical profiling demonstrating a malignancy which did not fit with any of the known classifications, thus describing a new tumour: microcystic tubulopapillary. Although some features of this new tumour were similar to solid pseudopapillary, acinar cell and even adenocarcinomas of the pancreas, it had a unique molecular profile in comparison to other forms of pancreatic tumours. This included wild type k-ras, loss of DPC4/SMAD4, deletion of exon 1 of p16INK4A and absence of p53 overexpression.8 The largest published case series to date is from Japan and reports data from 10 similar cases (five men, five women and mean age 58) of intraductal pancreatic tumours predominantly exhibiting a tubulopapillary growth pattern but without mucin secretion or acinar characteristics. Histology from tissue samples of each of the 10 patients was obtained and underwent immunohistochemical and mutational analyses. The authors concluded that the presence of specific pathognomonic features (solid obstructing tumour with dilated ducts, no mucin secretion, tubulopapillary growth pattern, uniform high grade atypia, frequent necrotic foci, strong CK7 and CK19 gene expression, absence of acinar differentiation/trypsin staining, negative MUC2/MUC5AC and fascin staining, negative for b-raf and k-ras mutations) justified the characterisation of ITPN as a separate classification of intraductal pancreatic neoplasm.9
Our case is another interesting and distinct case of ITPN and the first reported case of such a cancer arising as a secondary malignancy within a previously irradiated area. This is important as second malignancy as a late effect of radiation treatment for Hodgkins lymphoma is becoming a heightened risk as the treatment itself has led to a dramatic increase in survival of these patients. Sixty years ago life expectancy was approximately 3 years only for patients with Hodgkins disease.10 The advent of radical radiation and newer adjuvant chemotherapies such as adriamycin, bleomycin, vinblastine, dacarbazine to replace MOPP has greatly increased cure rates11 and consequently the observation of long-term side effects. It was in the 1970’s that secondary cancers first became a recognised side effect of treatment.12 Cases of secondary malignancies following intensive treatment for Hodgkin’s lymphoma are now well established in the literature13–15 and although it is mostly leukaemia that accounts for these, there are several studies which report a significantly higher risk of solid tumours in patients receiving radiotherapy16–18 and development depends on a number of factors, including length of follow-up, age and treatment given. Data from the early 1990’s suggest that 1 in 6 patients risk development of a second malignancy within 15 years of treatment.19 The Norwegian Cancer Registry reported in 1993 that 46% all solid tumours arose within or at the margin of a previously irradiated field20 and these data were reproduced by Birdwell et al in 1997, specifically for gastrointestinal tumours, establishing that 60% arose within or at the margin of a radiation field.21 Only very few pancreatic tumours were found within these cohorts but they were reported. This evidence serves to demonstrate the connection between this very rare pancreatic malignancy and the intensive radiotherapy given to treat Hodgkin’s disease.
Furthermore, this case adds to the small but growing database of knowledge we have about this diagnosis and also highlights the necessity for accurate and detailed histological examination and reporting of pathology specimens, especially in cases where the diagnosis is ambiguous or uncertain. Outcomes from these rare pancreatic tumours may be different to the poor prognosis that we have come to expect from the commoner invasive ductal adenocarcinomas. There is no clear, evidence based management strategy at present either. In this case it is debatable as to whether the initial course of gemcitabine was effective and although the oxaliplatin was given as a radiosensitiser prior to Yttrium-90 radioembolisation it, serendipitously, may have led to an ongoing response in its own right. Therefore, having a confirmed diagnosis would enable us to subcategorise this distinct aetiology, a mass separate epidemiological data and foster experience to guide treatment. This would allow for more realistic discussions with patients, expectations and a greater understanding of the natural history of intraductal tubulopapillary neoplasms of the pancreas.
Learning points.
-
▶
Rare diagnosis can have good prognoses.
-
▶
Diagnostic accuracy is paramount as management can be very different for different diseases.
-
▶
If unsure – continually re-review and reassess imaging and pathology.
-
▶
Anecdotal evidence can be useful especially to guide management of rare diseases.
-
▶
Long-term follow-up is important as second malignancies are increasingly a risk as survival rates following curative therapy for Hodgkin’s disease improve.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Cancer Research, UK http://www.cancerhelp.org.uk (accessed 1 August 2011)
- 2.Johns Hopkins Medicine: the Sol Goldman Pancreas Cancer Research Center Types of Pancreas Tumors. http://pathology.jhu.edu/pancreas/BasicTypes1.php (accessed 1 August 2011).
- 3.Mansfield A, Tafur A, Smithedajkul P, et al. Mayo Clinic experience with very rare exocrine pancreatic neoplasms. Pancreas 2010;39:972–5 [DOI] [PubMed] [Google Scholar]
- 4.Kloppel G, Hruban RH, Longnecker DS, et al. Ductal adenocarcinoma of the pancreas. In: Hamilton SR, Aaltonen LA, eds. WHO Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press; 2000:237–40 [Google Scholar]
- 5.Longnecker DS, Adler G, Hruban RH, et al. Intraductal mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, eds. WHO Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press; 2000:237–40 [Google Scholar]
- 6.Solcia E, Capella C, Kloppel G. Tumours of the Pancreas: Atlas of Tumour Pathology. Washington, DC: Armed Forces Institute of Pathology; 1997 [Google Scholar]
- 7.Klöppel G, Kosmahl M. Cystic lesions and neoplasms of the pancreas. The features are becoming clearer. Pancreatology 2001;1:648–55 [DOI] [PubMed] [Google Scholar]
- 8.Esposito I, Bauer A, Hoheisel JD, et al. Microcystic tubulopapillary carcinoma of the pancreas: a new tumour entity? Virchows Arch 2004;444:447–53 [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi H, Shimizu M, Ban S, et al. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2009;33:1164–72 [DOI] [PubMed] [Google Scholar]
- 10.Boyce J. Second Cancer after Hodgkin’s disease – the price of success? J Nat Cancer Inst 1993;85:4–5 [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA. The treatment of Hodgkin’s disease. Ann Oncol 1994;5 Suppl 2:17–21 [DOI] [PubMed] [Google Scholar]
- 12.Arseneau JC, Sponzo RW, Levin DL, et al. Nonlymphomatous malignant tumors complicating Hodgkin’s disease. Possible association with intensive therapy. N Engl J Med 1972;287:1119–22 [DOI] [PubMed] [Google Scholar]
- 13.Kaldor JM, Dau NE, Clarke EA, et al. Leukaemia following Hodhkins Disease. N Engl Med 1990;7:762–9 [Google Scholar]
- 14.Tucker MA, Coleman CN, Cox RS, et al. Risk of second cancers after treatment for Hodgkin’s disease. N Engl J Med 1988;318:76–81 [DOI] [PubMed] [Google Scholar]
- 15.Tester WJ, Kinsella TJ, Waller B, et al. Second malignant neoplasms complicating Hodgkin’s disease: the National Cancer Institute experience. J Clin Oncol 1984;2:762–9 [DOI] [PubMed] [Google Scholar]
- 16.van Leeuwen FE, Somers R, Taal BG, et al. Increased risk of lung cancer, non-Hodgkin’s lymphoma, and leukemia following Hodgkin’s disease. J Clin Oncol 1989;7:1046–58 [DOI] [PubMed] [Google Scholar]
- 17.Boivin JF, O’Brien K. Solid cancer risk after treatment of Hodgkin’s disease. Cancer 1988;61:2541–6 [DOI] [PubMed] [Google Scholar]
- 18.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med 1996;334:745–51 [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow AJ, Douglas AJ, Hudson GV, et al. Risk of second primary cancers after Hodgkin’s disease by type of treatment: analysis of 2846 patients in the British National Lymphoma Investigation. BMJ 1992;304:1137–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrahamsen JF, Andersen A, Hannisdal E, et al. Second malignancies after treatment of Hodgkin’s disease: the influence of treatment, follow-up time, and age. J Clin Oncol 1993;11:255–61 [DOI] [PubMed] [Google Scholar]
- 21.Birdwell SH, Hancock SL, Varghese A, et al. Gastrointestinal cancer after treatment of Hodgkin’s disease. Int J Radiat Oncol Biol Phys 1997;37:67–73 [DOI] [PubMed] [Google Scholar]