Abstract
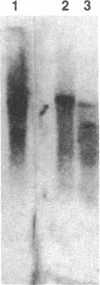
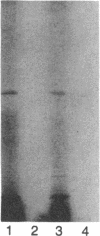
Galactosyltransferases constitute a family of enzymes, each member of which transfers galactose from UDPgalactose to a specific acceptor molecule, generating a specific galactose-acceptor linkage. Two synthetic oligonucleotides, 27mer and 21mer, were synthesized, based on the amino acid sequences of two peptides derived from bovine milk N-acetylglucosaminide (beta 1-4)galactosyltransferase (EC 2.4.1.90), and used as hybridization probes to isolate cDNA clones for galactosyltransferase from a bovine mammary gland cDNA library. One of the plasmids, designated pLbGT-1, contains an insert of about 3.7 kilobases that hybridizes to both of the probes and encodes the amino acid sequences of five peptides obtained from bovine milk (beta 1-4)galactosyltransferase. A second plasmid, designated pLbGT-2, contains an insert of about 4.1 kilobases that hybridizes to only the 27mer and that encodes a polypeptide containing the sequence of the carboxyl-terminal 120 residues identical to the peptide encoded by pLbGT-1; the rest of the protein sequence, however, does not contain known sequences from bovine galactosyltransferase. The two cDNAs contain a 3'-untranslated region of about 2.7 kilobases that includes two copies of the Alu-equivalent sequences. pLbGT-1 and pLbGT-2 hybridize to mRNAs of various sizes obtained from the bovine and rat mammary gland and the human mammary tumor cell line MCF-7, with the longest mRNA from each species being around 4.5 kilobases. The results show that pLbGT-1 is a cDNA clone for bovine (beta 1-4)galactosyltransferase, and pLbGT-2 encodes a protein that is structurally and may be functionally related to transferases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Beyer T. A., Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationships. Adv Enzymol Relat Areas Mol Biol. 1981;52:23–175. doi: 10.1002/9780470122976.ch2. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Castellino F. J., Vanaman T. C., Hill R. L. The complete amino acid sequence of bovine alpha-lactalbumin. J Biol Chem. 1970 Sep 10;245(17):4570–4582. [PubMed] [Google Scholar]
- Brew K., Hill R. L. Lactose biosynthesis. Rev Physiol Biochem Pharmacol. 1975;72:105–158. doi: 10.1007/BFb0031548. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. Comparison of the amino acid sequence of bovine alpha-lactalbumin and hens egg white lysozyme. J Biol Chem. 1967 Aug 25;242(16):3747–3749. [PubMed] [Google Scholar]
- Chatterjee S. K., Bhattacharya M., Barlow J. J. Murine monoclonal antibodies against galactosyltransferase from the ascites of ovarian cancer patients. Cancer Res. 1984 Dec;44(12 Pt 1):5725–5732. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dandekar A. M., Qasba P. K. Rat alpha-lactalbumin has a 17-residue-long COOH-terminal hydrophobic extension as judged by sequence analysis of the cDNA clones. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4853–4857. doi: 10.1073/pnas.78.8.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Roth S. Co-purification of galactosyltransferases from chick-embryo liver. Biochem J. 1985 Apr 15;227(2):573–582. doi: 10.1042/bj2270573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Brew K., Grant G. A., Bradshaw R. A., Lennarz W. J. Primary structural requirements for the enzymatic formation of the N-glycosidic bond in glycoproteins. Studies with natural and synthetic peptides. J Biol Chem. 1979 Oct 10;254(19):9747–9753. [PubMed] [Google Scholar]
- Hill R. L., Brew K. Lactose synthetase. Adv Enzymol Relat Areas Mol Biol. 1975;43:411–490. doi: 10.1002/9780470122884.ch5. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. DNA elements are asymmetrically joined during the site-specific recombination of kappa immunoglobulin genes. Science. 1985 May 10;228(4700):677–685. doi: 10.1126/science.3158075. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee S. C., Mawal R., Ebner K. E. Proteolytic conversion of the molecular forms of bovine milk galactosyltransferase. J Biol Chem. 1973 Nov 10;248(21):7565–7569. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Brew K. The preparation and characterization of two forms of bovine galactosyl transferase. Eur J Biochem. 1974 Oct 1;48(1):217–228. doi: 10.1111/j.1432-1033.1974.tb03759.x. [DOI] [PubMed] [Google Scholar]
- Qasba P. K., Safaya S. K. Similarity of the nucleotide sequences of rat alpha-lactalbumin and chicken lysozyme genes. Nature. 1984 Mar 22;308(5957):377–380. doi: 10.1038/308377a0. [DOI] [PubMed] [Google Scholar]
- Roth S. Are glycosyltransferases the evolutionary antecedents of the immunoglobulins? Q Rev Biol. 1985 Jun;60(2):145–153. doi: 10.1086/414313. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Shaper N. L., Shaper J. H., Meuth J. L., Fox J. L., Chang H., Kirsch I. R., Hollis G. F. Bovine galactosyltransferase: identification of a clone by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1573–1577. doi: 10.1073/pnas.83.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S. K., Brew K. A label selection procedure for determining the location of protein-protein interaction sites by cross-linking with bisimidoesters. Application to lactose synthase. J Biol Chem. 1981 May 10;256(9):4193–4204. [PubMed] [Google Scholar]
- Smith C. A., Brew K. Isolation and characteristics of galactosyltransferase from Golgi membranes of lactating sheep mammary glands. J Biol Chem. 1977 Oct 25;252(20):7294–7299. [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. R., Weiser M. M., Albini B., Schenck J. R., Rittenhouse H. G., Hirata A. A., Berger E. G. Co-purification of soluble human galactosyltransferase and immunoglobulins. Biochem Biophys Res Commun. 1982 Mar 30;105(2):737–744. doi: 10.1016/0006-291x(82)91496-6. [DOI] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferra F., Engh H., Hudson L., Kamholz J., Puckett C., Molineaux S., Lazzarini R. A. Alternative splicing accounts for the four forms of myelin basic protein. Cell. 1985 Dec;43(3 Pt 2):721–727. doi: 10.1016/0092-8674(85)90245-4. [DOI] [PubMed] [Google Scholar]