Abstract
Magnetic resonance elastography (MRE) is a noninvasive phase-contrast technique for estimating the mechanical properties of tissues by imaging propagating mechanical waves within the tissue. In this study, we hypothesize that changes in arterial wall stiffness, experimentally induced by formalin fixation, can be measured using MRE in ex vivo porcine aortas. In agreement with our hypothesis, the significant stiffness increase after sample fixation were clearly demonstrated by MRE and confirmed by mechanical testing. The results indicate that MRE can be used to examine the stiffness changes of the aorta. This study has provided evidence of the effectiveness of using MRE to directly assess the stiffness change in aortic wall. The results offer motivation to pursue MRE as a noninvasive method for the evaluation of arterial wall mechanical properties.
Keywords: MRI, Elastography, Aorta, Stiffness
1. Introduction
In recent years, great emphasis has been placed on the role of arterial stiffness in the development of cardiovascular diseases. Increased arterial stiffness has been found to be associated with increased morbidity and cardiovascular mortality in hypertensive patients [1,2]. Arterial walls stiffen with age. The most consistent and well-reported changes with age are luminal enlargement with wall thickening (remodeling) and stiffening at the level of large elastic arteries, namely arteriosclerosis [3]. Histologically, arteriosclerotic changes are recognized by alterations in the media. The principal structural change with aging is medial degeneration, which leads to progressive stiffening of the large elastic arteries [4]. Stiffening of large arteries results in various adverse hemodynamic consequences. Decreased compliance of the central vasculature alters arterial pressure and flow dynamics and impacts cardiac performance and coronary perfusion [5].
As arterial stiffness has been established as a cardiovascular risk factor, it has also emerged as a potential target for intervention. It is conceivable that reduction of arterial stiffness may become a major primary goal of treatment in particular patients at risk of cardiovascular disease [3]. There are a large number of studies reporting changes in arterial stiffness after various interventions, both pharmacological and nonpharmacological [5,6]. Early detection of arterial stiffness is important to better identify those at higher risk for subsequent cardiovascular outcomes and to modify their clinical course by utilizing appropriate medical and behavioral interventions. There is a need for a reliable, noninvasive method of detecting early disturbances in arterial stiffness at a time when therapeutic intervention is most beneficial.
The mechanics of the dynamic fluid-filled soft structure of the arteries are poorly understood and a major challenge for current techniques designed to assess arterial wall stiffness. Several noninvasive methods are currently used to assess arterial stiffness, the primary methods involving measurements of pulse wave velocity (PWV) or the augmentation index (AI). The main limitation of PWV and AI interpretation is that they are influenced by blood pressure. Reproducibility and operator dependence are barriers to their wide clinical use [4]. Hence, direct measurement of aortic elasticity is not readily available and no diagnostic gold standard has been established so far.
Two MR-based methods, phase-contrast MR angiography and MR elastography (MRE), have been used to evaluate the mechanical properties of the arterial wall. The feasibility of MR angiographic methods for the estimation of the time-dependent elastic modulus of healthy arteries using measurements of blood velocity during the cardiac cycle has been reported [7]. MRE is a noninvasive phase-contrast technique for estimating the mechanical properties of tissues by imaging propagating mechanical waves within the tissue. MRE has been applied to quantitatively assess the viscoelastic properties of many human tissues in vivo, including breast, brain, muscle, and liver by producing shear waves in these tissues [8–11].
Prior studies have shown MRE has the potential to evaluate the elastic properties of vessel walls using measurements of mechanical wave propagation in the fluid near the vessel walls [12,13]. Woodrum et al. reported the measurement of the elastic properties of ex vivo porcine aortas in control and hypertensive animals using MRE. The Young’s modulus-wall thickness product, a reflection of vascular stiffness, was higher in the hypertension group than in the control group (0.571 ± 0.080 vs. 0.419 ± 0.026 kPa-m) [14]. It is not clear that this change was due to changes in Young’s modulus or due to changes in the arterial wall thickness as there is an increase in intima and media thickness in hypertensive aortas. In this study, we hypothesize that changes in arterial wall stiffness itself, experimentally induced by formalin fixation, can be measured using MRE in ex vivo porcine aortas.
2. Materials and methods
2.1. Ex vivo porcine aorta
Five ex vivo porcine aortas were obtained from seven-month-old male domestic healthy pigs (105–136 kg) within 15 minutes of slaughter from a local commercial slaughterhouse. The aortas were immersed in 0.9% saline solution and preserved at room temperature prior to examination. A part of connective tissue was removed from the outside of the aorta and the side branches were tied off. The thickness and diameter of the aortas were measured with digital calipers. The length of the porcine aortas was approximately 20 cm with a diameter of approximately 11 mm and wall thickness of approximately 1.5 mm. Each fresh aorta was inserted into a single-channel transmit-receive (T/R) MR head coil under static pressure (20 mm of mercury) filled with normal saline. An electromechanical driver was applied to the vessel wall to generate mechanical waves within the aortas. The aortas were imaged using MRE. At the time of preparation, a small segment of the aorta (2.5 cm long) was also cut out for biomechanical testing. The biomechanical tests were performed at the same time as the MRE scans. The total time from death of the animal to completion of all examinations on the fresh aortas was approximately three to six hours. After the first MRE scan and biomechanical test, each individual aortic sample was immersed in 10% neutral buffered formalin (NBF) (Fisher Scientific Company L.L.C. Kalamazoo, MI) for 6 days. The initial volume ratio of formalin to tissue was 20:1. All aortic samples became stiff after formalin fixation. The fixed aortic samples were reexamined using the same MRE protocol and biomechanical test at room temperature.
2.2. Magnetic resonance elastography
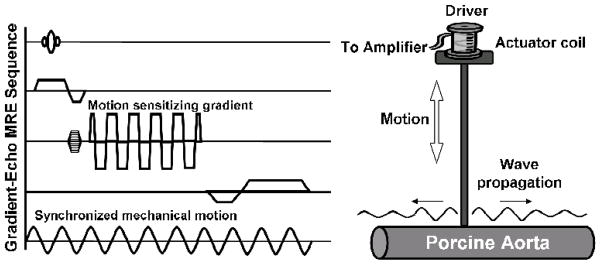
For all experiments, imaging was performed on a 1.5-T whole-body imager (GE Medical Systems, Waukesha, WI, USA) utilizing a single-channel T/R head coil. A two-dimensional gradient-echo MRE sequence was used for imaging with motion-sensitizing field gradients that were synchronized with the propagating mechanical waves to encode tissue displacement into the phase of the image data (15). Data acquisition parameters included the following: one slice of 5-mm thickness, 100-ms repetition time (TR), 256 × 64 acquisition matrix which was interpolated to a 256 × 256 matrix, 30° flip angle, and FOV of 35 cm. Five pairs of motion-encoding gradients were applied in the vertical direction. The porcine aorta filled with normal saline was inserted into the head coil with an electromechanical driver connected to the vessel wall. The electromechanical driver consisted of a coil of wire attached to the surface of one end of a rectangular plate of semirigid plastic. The other end of the rectangular plate was connected to a custom frame that was secured to the top of the head coil. A plastic rod was attached to the end of the rectangular plate closest to the driver coil. Alternating current at the desired frequency of vibration was applied to the coil via a function generator and amplifier triggered by the imaging sequence. This current created a magnetic field orthogonal to the main magnetic field of the scanner that caused the driver to try to align itself with the main magnetic field, thus deflecting the plate and causing the rod to oscillate vertically. The vertical motion was transmitted to the aortas via the rod and caused harmonic mechanical waves to propagate longitudinally along the aortic wall. The pulse sequence and experimental driver setup is shown in Figure 1. The imaging plane was along the longitudinal axis of the vessel. The motion trigger was adjusted during the scan to obtain images at 4 different phase offsets between the motion and the motion-encoding gradients equally spaced over one cycle of the motion. Because the wall of the aorta was too thin to be reliably imaged, the motion of the fluid adjacent to the vessel wall was used as a surrogate measure of the vibrations in the wall itself. The wavelengths in the fluid were calculated using peak-to-peak measurements of the propagating waves. The experiments were conducted using vibration frequencies of 200Hz. Preprocessing of the data consisted of unwrapping the phase (displacement) data, Fourier transforming the displacement data through the time offsets to extract the motion at the fundamental frequency of the vibration, and directional filtering to isolate wave propagation occurring along the vessel [16]. The wavelength of the motion was measured in the directionally filtered wave images. The reader who processed the MRE data was blinded to the results of mechanical testing. The mean Young’s modulus was calculated using the following expression, which was developed previously [12].
Fig. 1. The pulse sequence and experimental driver setup.
Schematic of a gradient-echo pulse sequence with an additional motion-sensitizing gradient which can be applied along any axis. By adjusting the phase delay between the motion and the motion-sensitizing gradients, the wave motion in the vessel can be imaged at different time points (left). The schematic diagram of the experimental setup for MR elastography shows the electromechanical driver in contact with a vessel. Vertical motion is transmitted to the vessel and causes harmonic mechanical waves to propagate longitudinally along the vessel wall (right)
| (1) |
where E is the Young’s modulus, t is the wall thickness of the aorta, ai is the interior aorta radius, ρ is the density of the interior fluid, λ is the wavelength, and f is the frequency of dynamic excitation.
2.3. Mechanical testing
The specimens of fresh and fixed aortas were mounted in a MTS 312 servohydraulic testing machine (MTS, Eden Prairie, MN) for uniaxial tensile testing. The width and thickness of the specimen strip and the slack length between the grips at the two ends were measured prior to testing. Each specimen was preloaded to 0.1 N, and then stretched at a rate of 2 mm/min. Samples were kept moist during testing with normal saline. Load and displacement data were collected at a sampling rate of 20Hz. Young’s modulus was calculated from the linear regression of tensile stress and strain curve at 5–50% strain range.
2.4. Statistical analysis
Continuous data were expressed as mean ± standard deviation. The Young’s modulus of ex vivo porcine aortas before and after formalin fixation both from MRE and mechanical testing were compared using paired Student’s t-test. A least-squares linear regression analysis was performed to compare the results of MRE with biomechanical testing. A p value less than 0.05 was considered to indicate a statistically significant difference. Statistical software (JMP 8.0; SAS, Cary, NC, USA) was used to perform the statistical analysis.
3. Results
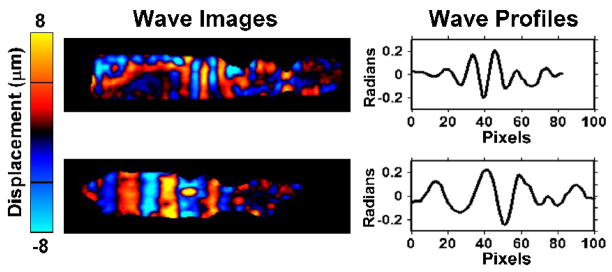
The propagating waves were well visualized in all of the aortas. Wavelength differences were observed between fresh and fixed aortas. The attenuation of the mechanical waves is apparent in the regions distant from the wave source and is more significant in the fresh aortas than in the fixed ones (Fig. 2). When no external motion is applied, no discernible waves were seen.
Fig. 2. MR Elastography of fresh and fixed ex vivo porcine aortas.
Wave images at exciting frequency of 200 Hz obtained with MRE in the fresh aorta (upper row) and the same sample after fixation (bottom row). The wavelength of the propagating waves can be seen to be much longer in the fixed aorta. This reflects a much higher stiffness value in the fixed aorta. The attenuation of the mechanical waves is more significant in the fresh aortas than in the fixed ones. The wavelength is measured from line profiles, as illustrated in the right column.
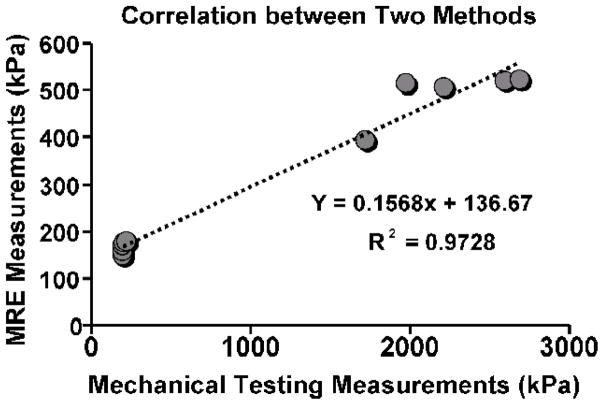
The mean thickness of the fresh aortas was 1.46 ± 0.09 mm while the fixed aortas were 1.51 ± 0.08 mm. The mean luminal diameter of the fresh aortas was 11.5 ± 0.3 mm while the fixed aortas were 11.0 ± 0.3 mm. The mean Young’s modulus of all of the fresh aortas for MRE was 165.0 ± 12.3 kPa (range 149.2–176.8 kPa) while the fixed-aorta mean stiffness was 491.3 ± 55.5 kPa (range 392.7–522.6 kPa). There was a significant increase in the stiffness of the fixed aortas compared to the fresh aortas (p<0.001). Biomechanical testing also demonstrated a significantly higher stiffness in the fixed aortas compared to the fresh aortas (p < 0.001). The mean Young’s modulus of the fresh aortas from mechanical testing was 202.4 ± 10.8 kPa (range 194.8–219.9 kPa) while the fixed-aorta mean stiffness was 2240.0 ± 406.8 kPa (range 1723.0–2690.0 kPa). The Young’s modulus of fresh and fixed aortas obtained from MRE and mechanical testing are illustrated in Figure 3. Typical stress-strain curves from the mechanical testing are shown in Figure 4. Regression analysis revealed a strong linear relationship of Young’s modulus (r2=0.972, p<0.001) between MRE and mechanical testing (Fig. 5).
Fig. 3. Comparison of Young’s modulus.
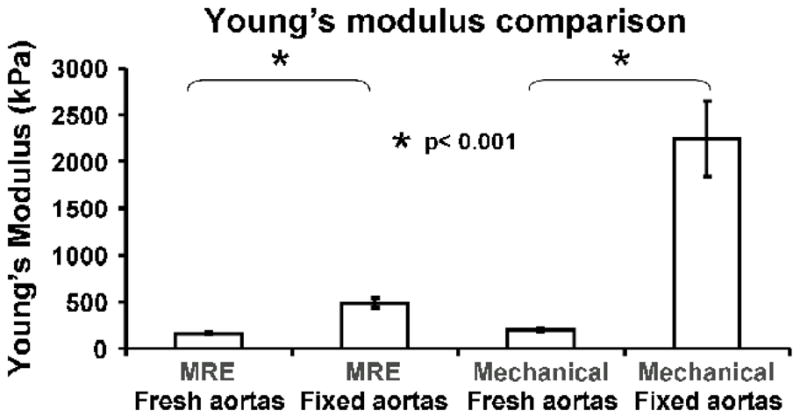
The Young’s modulus of fresh and fixed aortas obtained from MRE and mechanical testing.
Fig. 4. Typical stress-strain curves from the mechanical testing.
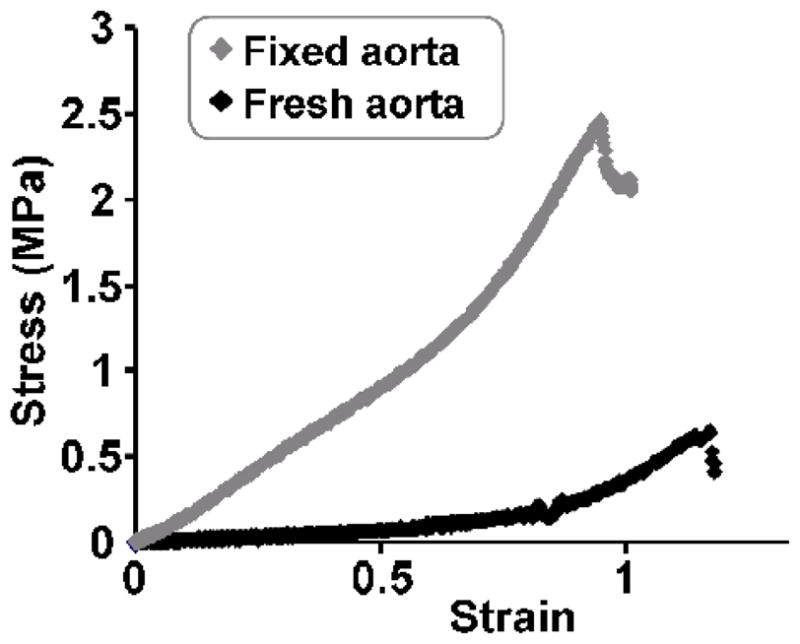
A representative plot of stress-strain curves of fresh and fixed aortas, as obtained during mechanical testing. Stiffness was calculated as the slope in the linear region.
Fig. 5. Relationship between MRE and mechanical testing.
A linear regression was performed between the MRE and mechanical testing Young’s modulus measurements. A strong correlation was observed (p < 0.001).
4. Discussion
This study reports the mechanical properties of samples from five ex vivo porcine aortas evaluated by MRE before and after formalin fixation. In agreement with our hypothesis, the significant stiffness changes after sample fixation were clearly demonstrated by MRE and confirmed by mechanical testing. The results indicate that MRE can be used to examine the stiffness changes of the aorta.
Magnetic resonance elastography is a novel MR-based imaging technique that noninvasively determines in vivo tissue stiffness [15,17]. MRE uses conventional MR imaging techniques with a superimposed oscillating motion-sensitizing gradient (usually in the frequency range of 50–500 Hz) to detect displacements produced by a phase-coupled shear excitation source. The shear wavelength is then used to calculate the material stiffness based on the principle that a stiffer material has a longer wavelength because the shear wave travels faster through the material [18]. It is well known that fluid does not support shear wave propagation, only pressure waves. In water, the speed of acoustic pressure waves is as fast as 1500 m/s, and the wavelength at the frequencies used for MRE is so long that MRE inversion algorithms are invalid. Woodrum et al. modeled the blood-filled vessel as a thin-wall fluid-filled elastic tube, where the displacement of the fluid due to motion of the vessel wall can be represented as 1D plane waves propagating along the longitudinal axis with a much slower speed, which makes it possible for MRE to measure the stiffness of the vessel [12]. These preliminary experiments demonstrated that externally produced harmonic mechanical waves can be clearly imaged with MRE in vessel models [12]. The wavelength at a constant frequency is a direct reflection of wave velocity and should change with changes in the mechanical properties of the vessel. Increased arterial stiffness results in an increased speed of wave propagation in the aortic wall. To characterize the ability of MRE to measure the wave velocity change within the thin-walled ex vivo vessel, and to demonstrate that the wave velocity is dependent on the associated wall mechanical properties, we changed the aortic wall stiffness by immersing the aortas into formalin while maintaining the morphology of the samples.
Fixatives often alter the cells or tissues on a molecular level to increase their mechanical strength or stability. This increased strength and rigidity can help preserve the shape and structure of the sample as it is processed [19]. 10% NBF remains the primary fixative used in clinical medicine. The usefulness of 10% NBF includes preventing extensive swelling or shrinkage of tissue, and only “hardening” of tissues after fixation [20]. Formalin fixation can decrease distensibility significantly in noncalcified arteries. Minimal changes of diameter of aorta may occur after fixation [21]. The elastic properties of the arterial wall material are estimated by Young’s incremental elastic modulus, which takes into account the thickness of the arterial wall. From the aforementioned formula (1), the measured Young’s modulus-wall thickness product is also related to the diameter of aortas. The potential small changes in the diameter and thickness of the aortas after formalin fixation do not affect the conclusion of this study since the changes in stiffness between fresh and fixed samples is far greater than the range of error produced by these changes in size.
Calculation of Young’s modulus assumes homogeneity of the arterial wall. The wall, however, is grossly inhomogeneous and thus the MRE and mechanical testing results reported here represent an effective modulus of the composite structure. MRE stiffness estimates using a finite elastic solid model can also be affected by multiple factors, including boundary conditions, geometry, and the amount of axial tension [22]. In this study, there was a strong linear relationship in Young’s modulus between MRE and mechanical testing. However, the Young’s modulus values between MRE and mechanical testing in the same sample were significantly different from each other. The substantial discrepancy between MRE and mechanical testing can be explained by the significant differences in the protocols and mechanical models used. For example, different geometrical sizes were used in the two testing methods and fluid loading was present in the aortas during MRE. Vascular tissues like the aorta exhibit nonlinear, anisotropic, frequency-dependent, and spatially nonuniform elastic behavior. The MRE estimate was based on microscopic strains induced by dynamic mechanical vibrations, whereas the mechanical test used much larger strains from quasistatic tension. Both the MRE and mechanical testing techniques ignored the effects of anisotropy. The wave propagation observed in MRE was due to the composite properties of the tissue, including the anisotropic properties, and only the wave speed along the length of the vessel was measured. Therefore, the reported MRE stiffness is further limited by not accounting for the truly 3D nature of the wave propagation and the geometry of the vessel. In the mechanical tests, uniaxial tension was applied to the sample and thus the circumferential or axial properties of the tissue were ignored.
The limitations of this study include relatively small sample size, the use of ex vivo aortic tissue, use of porcine rather than human arteries, and the fact that measurements were obtained in isobaric conditions, though the same static luminal pressure was applied during MRE testing. We did not investigate the relationship between luminal pressure and wall stiffness.
5. Conclusion
In this study, we examined ex vivo porcine aortas under isobaric conditions with the same luminal pressure for fresh and fixed aortas using MRE. The results showed a highly significant difference between fresh and fixed aortic stiffness using both MRE and mechanical tensile testing. This study has provided evidence of the effectiveness of using MRE to directly assess the stiffness change in aortic wall. The results offer motivation to pursue MRE as a noninvasive method for the evaluation of arterial wall mechanical properties without invasion of the artery itself. The application of this technique to in vivo human arteries, such as the aorta and femoral arteries, will be the focus of future studies. Further investigations will also include the feasibility of using MRE for studying arterial wall stiffness in elderly populations with arteriosclerosis and patients with hypertension.
Acknowledgments
This work has been supported by NIH grant EB001981, partly supported by the Natural Science Foundation of China (grant 81041013), International Collaboration Program from Chinese Ministry of Science and Technology (2010DFB30040), and a Program for Excellent Talents from Beijing city. The authors thank Diane M. Sauter for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 3.Izzo JL, Jr, Shykoff BE. Arterial stiffness: clinical relevance, measurement, and treatment. Rev Cardiovasc Med. 2001;2:29–34. 37–40. [PubMed] [Google Scholar]
- 4.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74:2257–62. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 6.Asmar R. Effect of antihypertensive agents on arterial stiffness as evaluated by pulse wave velocity: clinical implications. Am J Cardiovasc Drugs. 2001;1:387–97. doi: 10.2165/00129784-200101050-00008. [DOI] [PubMed] [Google Scholar]
- 7.Taviani V, Sutcliffe MP, Wong P, Li ZY, Young V, Graves MJ, et al. In vivo non-invasive high resolution MR-based method for the determination of the elastic modulus of arterial vessels. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5569–72. doi: 10.1109/IEMBS.2008.4650476. [DOI] [PubMed] [Google Scholar]
- 8.McKnight AL, Kugel JL, Rossman PJ, Manduca A, Hartmann LC, Ehman RL. MR elastography of breast cancer: preliminary results. AJR Am J Roentgenol. 2002;178:1411–7. doi: 10.2214/ajr.178.6.1781411. [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Lin Y, Han JC, Xi ZN, Shen H, Gao PY. Magnetic resonance elastography of brain tumors: preliminary results. Acta Radiol. 2007;48:327–30. doi: 10.1080/02841850701199967. [DOI] [PubMed] [Google Scholar]
- 10.Ringleb SI, Bensamoun SF, Chen Q, Manduca A, An KN, Ehman RL. Applications of magnetic resonance elastography to healthy and pathologic skeletal muscle. J Magn Reson Imaging. 2007;25:301–9. doi: 10.1002/jmri.20817. [DOI] [PubMed] [Google Scholar]
- 11.Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–13. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodrum DA, Romano AJ, Lerman A, Pandya UH, Brosh D, Rossman PJ, et al. Vascular wall elasticity measurement by magnetic resonance imaging. Magn Reson Med. 2006;56:593–600. doi: 10.1002/mrm.20991. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Chan QC, Li G, Lam EY, Yang ES. A study of femoral artery by twin drivers in magnetic resonance interference elastography. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2034–7. doi: 10.1109/IEMBS.2007.4352719. [DOI] [PubMed] [Google Scholar]
- 14.Woodrum DA, Herrmann J, Lerman A, Romano AJ, Lerman LO, Ehman RL. Phase-contrast MRI-based elastography technique detects early hypertensive changes in ex vivo porcine aortic wall. J Magn Reson Imaging. 2009;29:583–7. doi: 10.1002/jmri.21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–7. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 16.Manduca A, Lake DS, Kruse SA, Ehman RL. Spatio-temporal directional filtering for improved inversion of MR elastography images. Med Image Anal. 2003;7:465–73. doi: 10.1016/s1361-8415(03)00038-0. [DOI] [PubMed] [Google Scholar]
- 17.Kruse SA, Smith JA, Lawrence AJ, Dresner MA, Manduca A, Greenleaf JF, et al. Tissue characterization using magnetic resonance elastography: preliminary results. Phys Med Biol. 2000;45:1579–90. doi: 10.1088/0031-9155/45/6/313. [DOI] [PubMed] [Google Scholar]
- 18.Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5:237–54. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 19.Grizzle WE. Special symposium: fixation and tissue processing models. Biotech Histochem. 2009;84:185–93. doi: 10.3109/10520290903039052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesnick IE, Mason JT, O’Leary TJ, Fowler CB. Elevated Pressure Improves the Rate of Formalin Penetration while Preserving Tissue Morphology. J Cancer. 2010;1:178–83. doi: 10.7150/jca.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JC, Siegel RJ, Demer LL. Effect of calcification and formalin fixation on in vitro distensibility of human femoral arteries. Am Heart J. 1993;125:344–9. doi: 10.1016/0002-8703(93)90010-7. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Ringleb SI, Manduca A, Ehman RL, An KN. Differential effects of pre-tension on shear wave propagation in elastic media with different boundary conditions as measured by magnetic resonance elastography and finite element modeling. J Biomech. 2006;39:1428–34. doi: 10.1016/j.jbiomech.2005.04.009. [DOI] [PubMed] [Google Scholar]