Abstract
Objective
To demonstrate varicella zoster virus (VZV) infection in an asymptomatic extracranial (temporal) artery in a patient with ischemic optic neuropathy produced by VZV vasculopathy in whom the pathological changes were mistakenly identified as giant cell arteritis.
Design
Case report.
Setting
Teaching hospital, pathology and virology laboratory.
Patient
An 80-year-old man with left ophthalmic distribution zoster who developed left ischemic optic neuropathy.
Intervention
An ipsilateral temporal artery biopsy revealed inflammation that was mistakenly identified as giant cell arteritis. The patient was initially treated with steroids but his condition did not improve. When the diagnosis of VZV vasculopathy was confirmed virologically and the patient was treated with intravenous acyclovir, his vision improved.
Results
Pathological and virological studies provided proof of VZV vasculopathy in the asymptomatic temporal artery. Varicella zoster virus antigen was abundant in arterial adventitia and scattered throughout the media. With intravenous antiviral therapy, the patient’s vision improved.
Conclusion
Although in previously studied patients who died of chronic VZV vasculopathy after 10 to 12 months, VZV antigen was present exclusively in the intima, collective analyses of chronic cases and the asymptomatic VZV-infected temporal artery suggest that virus enters arteries through the adventitia and spreads transmurally to the intima.
Varicella Zoster Virus (VZV) vasculopathy produces transient ischemic attacks and stroke. Disease may be acute, but is usually chronic and protracted. Combined pathological and virological analyses of arteries in patients who died of VZV vasculopathy have revealed productive virus infection in a thickened arterial intima.1,2 The pathogenesis of disease is unclear. Cerebral arteries may become infected hematogenously. Alternatively, after reactivation, virus may spread transaxonally along ganglionic afferent fibers and enter arteries through the adventitia. We had the unique opportunity to study a patient with ischemic optic neuropathy due to VZV vasculopathy who had a temporal artery biopsy to rule out giant cell arteritis (GCA). Analysis of virus distribution in the asymptomatic temporal artery combined with virological findings in protracted fatal cases of VZV vasculopathy suggest that the virus enters cerebral arteries first in the adventitia followed by transmural migration to the thickened intima where productive infection continues.
REPORT OF A CASE
In February 2010, an 80-year-old man developed left ophthalmic distribution zoster that was treated with oral valacyclovir (1 g, 3 times daily for 1 week). One month later, he developed sudden painless loss of vision in the left eye. He denied headache, scalp tenderness, or jaw claudication. Findings of neurological examination were normal except for left eye visual acuity, which was reduced to counting fingers at 1-foot distance, an afferent pupillary defect, and 50% reduction in red saturation. Funduscopic examination showed no cherry-red spots, optic nerve pallor, retinal edema, or increased intraocular pressure. The erythrocyte sedimentation rate was 32 mm/h, and the C-reactive protein level was 16 mg/L (to convert to nanomoles per liter, multiply by 9.524). The cerebrospinal fluid (CSF) contained 5 white blood cells; the level of CSF protein was 51 mg/100 mL; the IgG index was 0.3 (reference range, 0.3–0.7); and there were no oligoclonal bands. Polymerase chain reaction analysis of CSF did not reveal amplifiable VZV or herpes simplex virus DNA; the CSF was also sent for testing for anti-VZV antibody. Brain magnetic resonance imaging with sections at multiple levels of the optic nerve revealed occlusion of the left ophthalmic artery without evidence of restricted diffusion or subacute infarction. Fluorescein angiography revealed left optic disc leakage with no choroidal perfusion nasally. Magnetic resonance angiography revealed no flow in the left ophthalmic artery; all other intracranial and extracranial arteries were normal. The patient was immediately treated empirically for GCA with a single dose of methylprednisolone (250 mg intravenously). Owing to his recent history of left-sided ophthalmic-distribution zoster, the diagnosis of VZV vasculopathy with acute occlusion of the ipsilateral ophthalmic artery was also considered; the next day, he was also treated with oral prednisone (80 mg daily) and intravenous acyclovir (10 mg/kg every 8 hours).
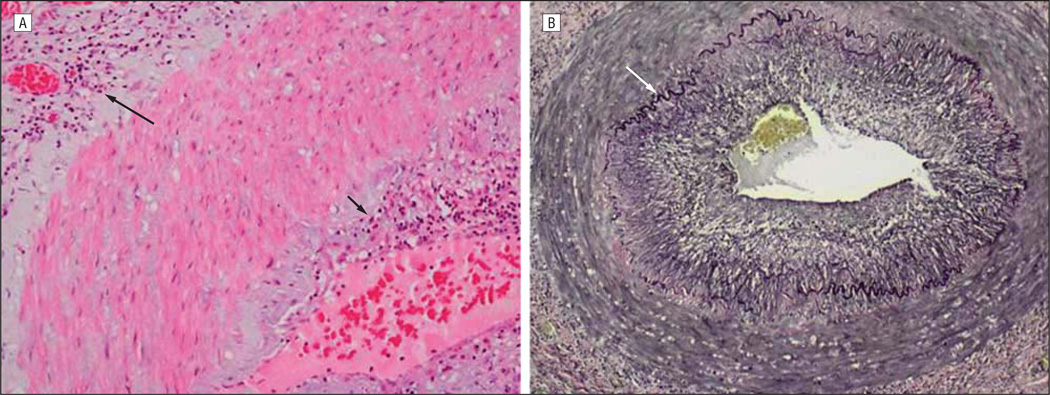
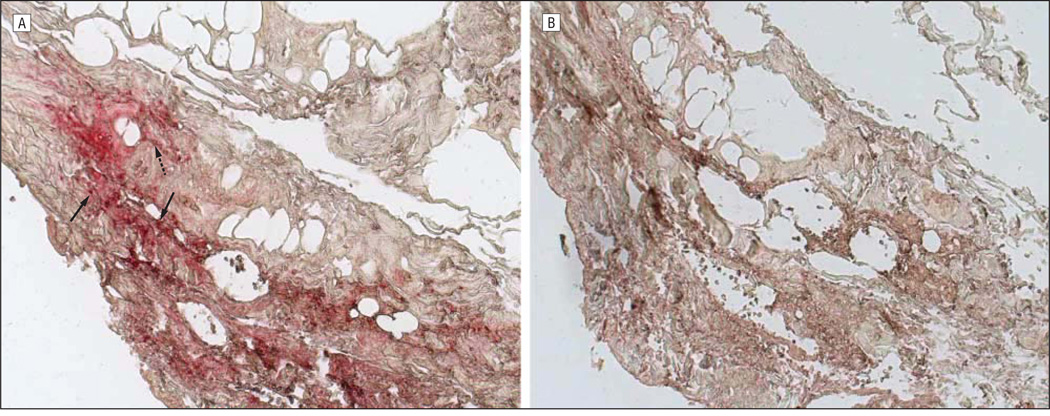
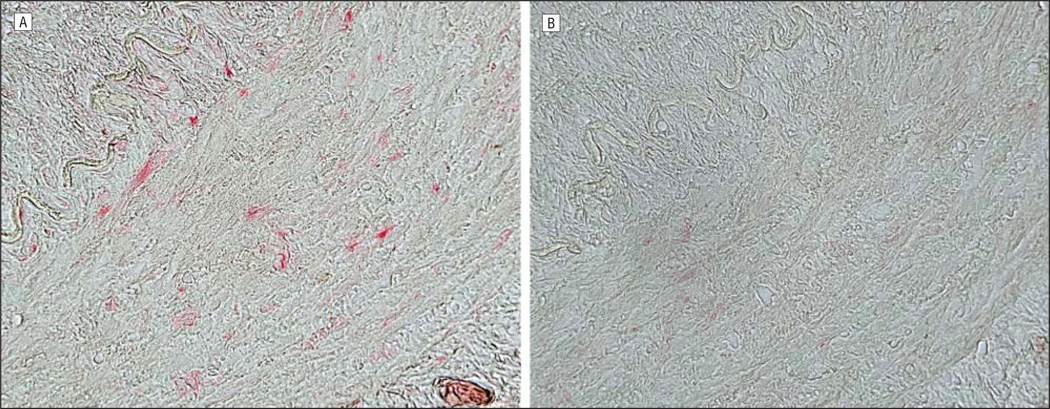
On the third hospital day, a temporal artery biopsy revealed inflammation of the intima and adventitia (Figure 1A) with eosinophils, lymphocytes, and multinucleated giant cells; the adventitia contained a lymphocytic infiltrate, with focal granulomatous inflammation of the vasa vasorum. Necrosis and vascular thrombosis were absent. The intima was thickened in areas with the most severe inflammation. The internal elastic lamina was almost completely intact (Figure 1B), with less than 5% to 10% of the circumferential diameter disrupted despite adjacent severe intimal inflammation. No phagocytosis of elastic lamina fragments was identified. Because some of these findings were consistent with GCA, intravenous acyclovir was discontinued after an initial 4-day course, although he continued to take oral prednisone (80 mg daily). Ten days later, analysis of CSF for antiviral antibody revealed anti-VZV IgM and IgG antibody but not anti–herpes simplex virus antibody. The serum to CSF ratio of anti-VZV IgM antibody was markedly reduced (4.6), as was the serum to CSF ratio of anti-VZV IgG antibody (3.6) compared with ratios for total IgG (344) and albumin (86), indicative of intrathecal synthesis of both anti-VZV IgM and anti-VZV IgG. Oral prednisone treatment was immediately reduced to 40 mg daily and tapered by 10 mg per week, and the patient received a 14-day course of intravenous acyclovir (10 mg/kg every 8 hours) followed by oral valacyclovir (1 g twice daily). Eight weeks after acute vision loss, the patient’s visual acuity had improved to 20/50. Oral valacyclovir was continued with planned close observation. The erythrocyte sedimentation rate was now 16 and C-reactive protein was 2.0. Subsequent immunohistochemistry on the temporal artery biopsy specimen revealed abundant VZV antigen in the adventitia (Figure 2A) and lesser amounts in the media (Figure 3A).
Figure 1.
Cross-section of the temporal artery examined on day 3 of patient’s hospitalization. Note the maximal inflammation in the intima (short arrow) and adventitia (long arrow), with relative sparing of the media (hematoxylin-eosin, original magnification ×200) (A) and the nearly intact, black-colored internal elastic lamina (arrow), despite the nearby intense intimal and adventitial inflammation (Verhoeff van Gieson stain for elastic fibers, original magnification ×100) (B).
Figure 2.
Immunohistochemical analysis of the temporal artery. Note varicella zoster virus (VZV) antigen (red) in the nucleus and cytoplasm (solid arrows) and cytoplasm alone (dashed arrow) of cells in the arterial adventitia (A; original magnification ×200) not seen with normal rabbit serum (B). Sections of the temporal artery were deparaffinized and incubated with 10% normal sheep serum in phosphate-buffered saline (PBS) for 1 hour at room temperature. To prevent nonspecific binding, primary antibodies were adsorbed with normal human liver powder for 30 minutes and again for 20 hours at 4°C. Sections were then incubated with polyclonal antibodies raised against VZV open reading frame 63 protein (1:1000 dilution) or normal rabbit serum (1:1000 dilution), rinsed with PBS and incubated with a 1:300 dilution of biotinylated goat antirabbit IgG in PBS containing 5% normal sheep serum, washed 3 times in PBS, incubated with alkaline phosphatase–conjugated streptavidin (1:100 dilution), and washed 3 times with PBS. The color reaction was developed for 5 to 30 minutes with fresh fuchsin substrate system. Levamisole was added to the color reaction to block endogenous phosphatase. Uninfected and VZV-infected BSC-1 cells were used as controls (not shown).
Figure 3.
Immunohistochemical analysis of the temporal artery as described in Figure 2. Note varicella zoster virus antigen scattered throughout the nucleus and cytoplasm of cells in the arterial wall (media) (A; original magnification ×400) not seen with normal rabbit serum (B).
COMMENT
Herein, we describe a case of ophthalmic distribution zoster followed 1 month later by ischemic optic neuropathy. The diagnosis of VZV vasculopathy was verified by the presence of anti-VZV antibody in CSF with reduced serum to CSF ratios of anti-VZV IgG and anti-VZV IgM antibody compared with ratios of albumin or total IgG. Importantly, the presence of both anti-VZV IgG and anti-VZV IgM antibodies indicated chronic active infection. The superiority of detection of antiviral antibody in CSF in the absence of amplifiable VZV DNA has been shown repeatedly in VZV vasculopathy.3 Furthermore, although vision loss began weeks before the presence of anti-VZV antibody was revealed, our patient’s vision improved from finger counting to 20/50 after 2 weeks of intravenous antiviral treatment. Varicella zoster virus vasculopathy has been successfully treated even after 6 months of disease.4 This case adds to uncommon earlier reports of VZV small-vessel vasculopathy in the eye that involved the central retinal5 and posterior ciliary4 arteries.
Because the patient was elderly and developed acute loss of vision, a temporal artery biopsy was performed initially to rule out GCA. Pathological changes revealed an inflammatory infiltrate, almost exclusively in the intima and adventitia, composed of lymphocytes, eosinophils, and occasional neutrophils, giant cell formation, a thickened intima, and minimal involvement of the arterial wall (media). While an inflammatory response with giant cells and thickened intima are seen in both GCA and VZV vasculopathy, inflammation is characteristically maximal in the media in GCA,6,7 whereas inflammation in our patient was greatest in the intima and adventitia, with relative sparing of the media. Furthermore, Verhoeff van Gieson staining in our patient’s temporal artery revealed that less than 10% of the internal elastic membrane was disrupted, unlike the targeted destruction of the elastic lamina seen in typical GCA.8 Finally, the detection of VZV antigen verified the diagnosis of VZV vasculopathy in the temporal artery.
A number of remarkable features emerge from analysis of this case. First, the development of VZV vasculopathy in an extracranial artery is noteworthy. There are only other 2 other cases of VZV vasculopathy affecting arteries that are not intracranial: one of a patient with spinal cord infarction due to VZV vasculopathy9 and another case of varicella in an adult who developed stroke and peripheral arterial thromboses.10 Second, the serendipitous biopsy of the temporal artery before any clinical symptoms or signs developed corresponding to the temporal artery revealed that viral antigen was most abundant in the arterial adventitia, with lesser amounts in the media. In contrast, viral antigen was seen exclusively in the thickened intimal layer in fatal cases of VZV vasculopathy that progressed for 10 to 12 months before death.1,2 Although additional cases of VZV vasculopathy need to be studied, it appears that early in the course of productive arterial infection, as seen in our patient, the virus enters the artery through the adventitia, presumably after transaxonal spread via ganglionic afferent fibers. Thereafter, transmural migration occurs, with productive infection continuing in the thickened intima.
Acknowledgments
Funding/Support: This study was supported in part by the Public Health Service grants AG006127 (Dr Gilden), AG032958 (Drs Gilden, Cohrs, and Mahalingam), and NS067070 (Dr Nagel) from the National Institutes of Health.
Footnotes
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Salazar, Russman, Nagel, Cohrs, Mahalingam, and Gilden. Acquisition of data: Salazar, Russman, Nagel, Cohrs, Mahalingam, Schmid, Kleinschmidt-DeMasters, VanEgmond, and Gilden. Analysis and interpretation of data: Russman, Nagel, Cohrs, Mahalingam, Schmid, Kleinschmidt-DeMasters, VanEgmond, and Gilden. Drafting of the manuscript: Salazar, Russman, Nagel, Cohrs, Mahalingam, Schmid, Kleinschmidt-DeMasters, VanEgmond, and Gilden. Critical revision of the manuscript for important intellectual content: Nagel. Administrative, technical, and material support: Salazar, Russman, Nagel, Cohrs, Mahalingam, Schmid, Kleinschmidt-DeMasters, and Gilden.
Financial Disclosure: None reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the view of the Centers for Disease Control and Prevention.
Additional Contributions: Marina Hoffman, BA, provided editorial assistance, and Cathy Allen provided manuscript preparation and formatting assistance.
REFERENCES
- 1.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology. 1996;47:1441–1446. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 2.Case Records of the Massachusetts General Hospital. Case records of the Massachusetts General Hospital: weekly clinicopathological exercises case 5-1995: a 73-year-old man with focal brain lesions and peripheral-nerve disease. N Engl J Med. 1995;332(7):452–459. doi: 10.1056/NEJM199502163320708. [DOI] [PubMed] [Google Scholar]
- 3.Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68(13):1069–1073. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- 4.Gilden DH, Lipton HL, Wolf JS, et al. Two patients with unusual forms of varicella-zoster virus vasculopathy. N Engl J Med. 2002;347(19):1500–1503. doi: 10.1056/NEJMoa020841. [DOI] [PubMed] [Google Scholar]
- 5.Hall S, Carlin L, Roach ES, McLean WT., Jr Herpes zoster and central retinal artery occlusion. Ann Neurol. 1983;13(2):217–218. doi: 10.1002/ana.410130226. [DOI] [PubMed] [Google Scholar]
- 6.Bruetsch WL. Giant cell arteritis (temporal arteritis, cranial arteritis, granulomatous angiitis) In: Minckler J, editor. Pathology of the Nervous System. Vol 2. New York, NY: McGraw-Hill Book Co; 1971. pp. 1456–1468. [Google Scholar]
- 7.Chatelain D, Duhaut P, Schmidt J, et al. Groupe de Recherche sur l’Artérite à Cellules Géantes. Pathological features of temporal arteries in patients with giant cell arteritis presenting with permanent visual loss. Ann Rheum Dis. 2009;68(1):84–88. doi: 10.1136/ard.2007.084947. [DOI] [PubMed] [Google Scholar]
- 8.Ashton-Key M, Gallagher PJ. Surgical pathology of cranial arteritis and polymyalgia rheumatica. Baillieres Clin Rheumatol. 1991;5(3):387–404. doi: 10.1016/s0950-3579(05)80061-5. [DOI] [PubMed] [Google Scholar]
- 9.Orme HT, Smith AG, Nagel MA, Bert RJ, Mickelson TS, Gilden DH. VZV spinal cord infarction identified by diffusion-weighted MRI (DWI) Neurology. 2007;69(4):398–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- 10.Massano J, Ferreira D, Toledo T, Mansilha A, Azevedo E, Carvalho M. Stroke and multiple peripheral thrombotic events in an adult with varicella. Eur J Neurol. 2008;15(10):e90–e91. doi: 10.1111/j.1468-1331.2008.02267.x. [DOI] [PubMed] [Google Scholar]