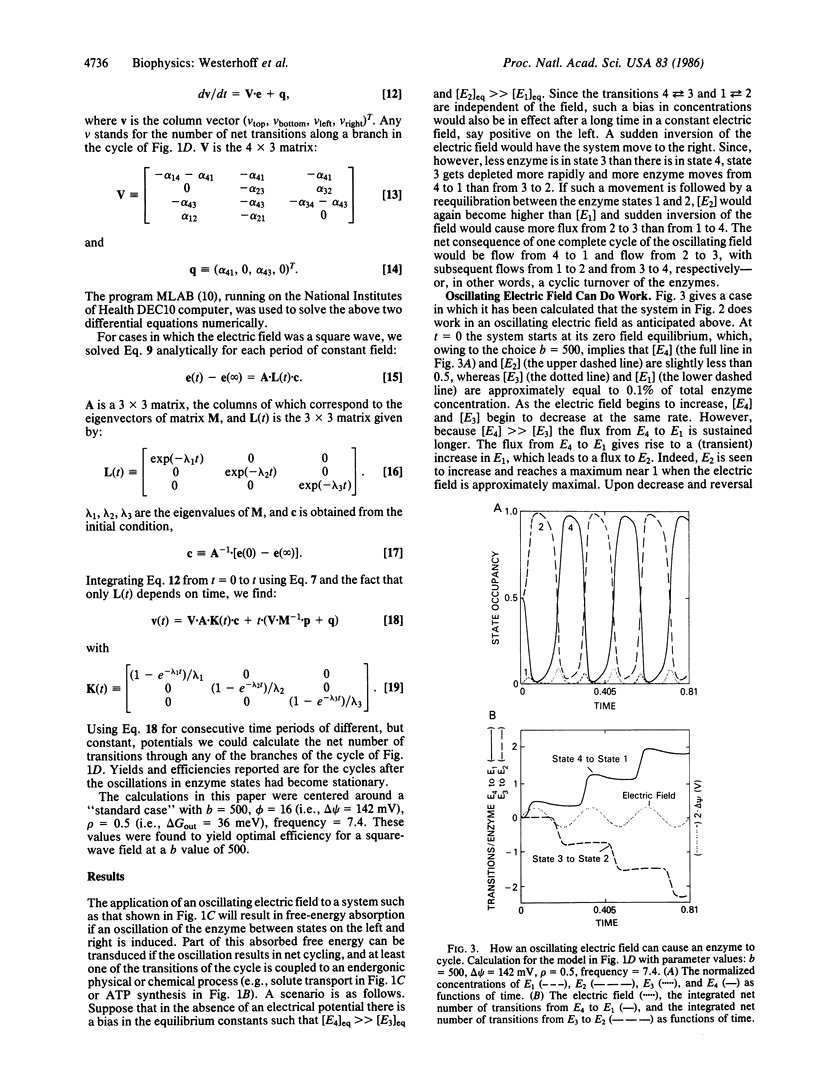
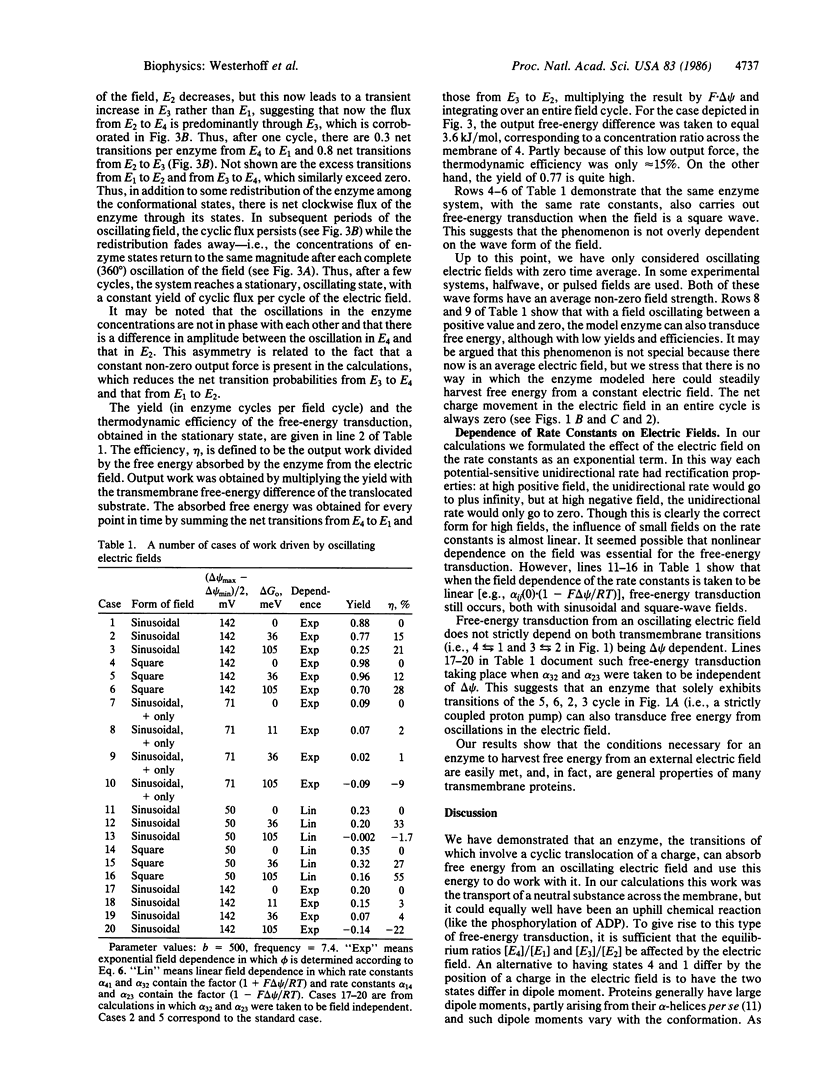
Abstract
Recently, it has been demonstrated that free energy from an alternating electric field can drive the active transport of Rb+ by way of the Na+, K+-ATPase. In the present work, it is shown why many transmembrane enzymes can be expected to absorb free energy from an oscillating electric field and transduce that to chemical or transport work. In the theoretical analysis it turned out to be sufficient that (i) the catalytic process be accompanied by either net or cyclic charge translocation across the membrane and (ii) the stability of the enzyme states involved be asymmetric. Calculations based on a four-state model reveal that free-energy transduction occurs with sinusoidal, square-wave, and positive-only oscillating electric fields and for cases that exhibit either linear or exponential field-dependent rate constants. The results suggest that in addition to oscillating electric field-driven transport, the proposed mechanism can also be used to explain, in part, the "missing" free energy term in the cases in which ATP synthesis has been observed with insufficient transmembrane proton electrochemical potential difference.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. W. Energy transfer and molecular switching II. Muscle contraction and enzymatic reactions. J Theor Biol. 1982 Nov 21;99(2):293–307. doi: 10.1016/0022-5193(82)90006-6. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Fuchs R. T., Krulwich T. A. Oxidative phosphorylation by isolated membrane vesicles from Bacillus megaterium and its uncoupler-resistant mutant derivative. J Biol Chem. 1983 Jan 10;258(1):35–37. [PubMed] [Google Scholar]
- Hol W. G., Halie L. M., Sander C. Dipoles of the alpha-helix and beta-sheet: their role in protein folding. Nature. 1981 Dec 10;294(5841):532–536. doi: 10.1038/294532a0. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Zoratti M., Azzone G. F. Molecular slipping in redox and ATPase H+ pumps. Biochim Biophys Acta. 1983 May 27;723(2):317–321. doi: 10.1016/0005-2728(83)90131-7. [DOI] [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Activation of electrogenic Rb+ transport of (Na,K)-ATPase by an electric field. J Biol Chem. 1984 Jun 10;259(11):7155–7162. [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Stimulation of a ouabain-sensitive Rb+ uptake in human erthrocytes with an external electric field. J Membr Biol. 1983;74(3):191–201. doi: 10.1007/BF02332123. [DOI] [PubMed] [Google Scholar]
- Skulachev V. P. The localized delta muH+ problem. The possible role of the local electric field in ATP synthesis. FEBS Lett. 1982 Sep 6;146(1):1–4. doi: 10.1016/0014-5793(82)80692-3. [DOI] [PubMed] [Google Scholar]
- Slater E. C., Berden J. A., Herweijer M. A. A hypothesis for the mechanism of respiratory-chain phosphorylation not involving the electrochemical gradient of protons as obligatory intermediate. Biochim Biophys Acta. 1985 Aug 1;811(3):217–231. doi: 10.1016/0304-4173(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Voltage modulation of membrane permeability and energy utilization in cells. Biosci Rep. 1983 Jun;3(6):487–505. doi: 10.1007/BF01120693. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Chen Y. Stochastic free energy transduction. Proc Natl Acad Sci U S A. 1985 May;82(10):3222–3226. doi: 10.1073/pnas.82.10.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff H. V., Melandri B. A., Venturoli G., Azzone G. F., Kell D. B. A minimal hypothesis for membrane-linked free-energy transduction. The role of independent, small coupling units. Biochim Biophys Acta. 1984 Dec 17;768(3-4):257–292. doi: 10.1016/0304-4173(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Wilhelm R. H., Sweed N. H. Parametric Pumping: Separation of Mixture of Toluene and n-Heptane. Science. 1968 Feb 2;159(3814):522–524. doi: 10.1126/science.159.3814.522. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Sakabe K., Matsubara Y., Ota T. Oscillation of electrical potential in a porous membrane doped with glycerol alpha-monooleate induced by an Na+/K+ concentration gradient. Biophys Chem. 1984 Aug;20(1-2):107–109. doi: 10.1016/0301-4622(84)80010-1. [DOI] [PubMed] [Google Scholar]


