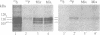Abstract
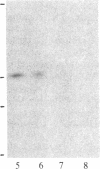
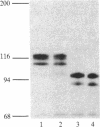
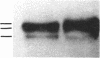
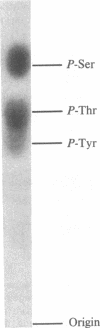
In vivo labeling with [35S]cysteine has identified three transmembrane forms of the rat hepatic polymeric IgA receptor: (i) a 105-kDa core glycosylated precursor; (ii) a terminally glycosylated 116-kDa intermediate; and (iii) a mature 120-kDa form. In the current study we show that the 120-kDa form is phosphorylated. After in vivo labeling with [32P]orthophosphate, all receptor forms were immunoprecipitated from hepatic total microsomes (TM) (with an antireceptor antiserum), separated by NaDodSO4/PAGE, and detected by autoradiography. The 120-kDa form was selectively phosphorylated, whereas the 116- and 105-kDa forms incorporated no detectable 32P. To determine the topology of the phosphorylation sites, hepatic TM isolated from rats labeled in vivo with either [35S]cysteine or [32P]orthophosphate were treated with trypsin. TM were solubilized and receptors were immunoprecipitated from lysates. With increasing trypsin concentrations, the [35S]cysteine-labeled receptor triplet was degraded to a trypsin-resistant doublet of approximately 95 and 85 kDa, indicating that approximately 20 kDa was removed from the receptor endodomain by trypsin. The same treatment removed all detectable 32P from labeled receptors. Furthermore, no 32P was detected in the 80-kDa biliary form of the receptor. Serine was identified as the only phosphorylated residue in acid hydrolysates of 32P-labeled immunoprecipitated receptor. These findings indicate that (i) the 120-kDa form is the only phosphorylated species of the receptor; and (ii) the phosphorylated residues are serine(s) located in the endodomain of the protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod N. Phosphoproteins of adenovirus 2. Virology. 1978 Jun 15;87(2):366–383. doi: 10.1016/0042-6822(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Pike L. J., Cerione R. A., Staniszewski C., Yoshimasa T., Codina J., Caron M. G., Lefkowitz R. J. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985 Jun 10;260(11):7094–7101. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carlin C. R., Phillips P. D., Knowles B. B., Cristofalo V. J. Diminished in vitro tyrosine kinase activity of the EGF receptor of senescent human fibroblasts. Nature. 1983 Dec 8;306(5943):617–620. doi: 10.1038/306617a0. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Hodgson H. J., McPherson A., Dive C., Vaerman J. P. Selective transport of polymeric immunoglobulin A in bile. Quantitative relationships of monomeric and polymeric immunoglobulin A, immunoglobulin M, and other proteins in serum, bile, and saliva. J Clin Invest. 1982 Aug;70(2):230–241. doi: 10.1172/JCI110610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K., Mamon J. F. Phosphorylation of a membrane receptor for glycoproteins. Possible transmembrane orientation of the chicken hepatic lectin. J Biol Chem. 1982 Dec 25;257(24):15156–15161. [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Fewtrell C., Davis C. L., Metzger H. Phosphorylation of the receptor of immunoglobulin E. Biochemistry. 1982 Apr 27;21(9):2004–2010. doi: 10.1021/bi00538a005. [DOI] [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to a positively charged membrane filter. Anal Biochem. 1982 Aug;124(2):396–405. doi: 10.1016/0003-2697(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Hanson L. A., Ahlstedt S., Andersson B., Carlsson B., Cole M. F., Cruz J. R., Dahlgren U., Ericsson T. H., Jalil F., Khan S. R. Mucosal immunity. Ann N Y Acad Sci. 1983 Jun 30;409:1–21. doi: 10.1111/j.1749-6632.1983.tb26855.x. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., DeGennaro L. J., Greengard P. Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J Biol Chem. 1981 Feb 10;256(3):1482–1488. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Kleinman R. E., Harmatz P. R., Walker W. A. The liver: an integral part of the enteric mucosal immune system. Hepatology. 1982 May-Jun;2(3):379–384. doi: 10.1002/hep.1840020315. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemaître-Coelho I., Altamirano G. A., Barranco-Acosta C., Meykens R., Vaerman J. P. In vivo experiments involving secretory component in the rat hepatic transfer of polymeric IgA from blood into bile. Immunology. 1981 Jun;43(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982 Oct 10;257(19):11816–11821. [PubMed] [Google Scholar]
- Mostov K. E., Kraehenbuhl J. P., Blobel G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlans E., Peppard J. V., Payne A. W., Fitzharris B. M., Mullock B. M., Hinton R. H., Hall J. G. Comparative aspects of the hepatobiliary transport of IgA. Ann N Y Acad Sci. 1983 Jun 30;409:411–427. doi: 10.1111/j.1749-6632.1983.tb26886.x. [DOI] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A., White M. F., Kahn C. R. Predominance of tyrosine phosphorylation of insulin receptors during the initial response of intact cells to insulin. J Biol Chem. 1985 Jun 10;260(11):7131–7136. [PubMed] [Google Scholar]
- Papermaster D. S., Converse C. A., Zorn M. Biosynthetic and immunochemical characterization of large protein in frog and cattle rod outer segment membranes. Exp Eye Res. 1976 Aug;23(2):105–115. doi: 10.1016/0014-4835(76)90194-9. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Bowen-Pope D. F., Ross R., Krebs E. G. Characterization of platelet-derived growth factor-stimulated phosphorylation in cell membranes. J Biol Chem. 1983 Aug 10;258(15):9383–9390. [PubMed] [Google Scholar]
- Renston R. H., Jones A. L., Christiansen W. D., Hradek G. T., Underdown B. J. Evidence for a vesicular transport mechanism in hepatocytes for biliary secretion of immunoglobulin A. Science. 1980 Jun 13;208(4449):1276–1278. doi: 10.1126/science.7375938. [DOI] [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Schwartz A. L. Phosphorylation of the human asialoglycoprotein receptor. Biochem J. 1984 Oct 15;223(2):481–486. doi: 10.1042/bj2230481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. I. Kinetic studies of its intracellular forms. J Cell Biol. 1985 Apr;100(4):1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. II. Localization of its intracellular forms by cell fractionation studies. J Cell Biol. 1985 Apr;100(4):1255–1261. doi: 10.1083/jcb.100.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Nakane P. K., Brown W. R. Ultrastructural events in the translocation of polymeric IgA by rat hepatocytes. J Immunol. 1982 Mar;128(3):1181–1187. [PubMed] [Google Scholar]
- Takahashi T., Nakada H., Okumura T., Sawamura T., Tashiro Y. Phosphorylation of the rat hepatocyte asialoglycoprotein receptor. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1054–1060. doi: 10.1016/0006-291x(85)90292-x. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Wang S. Y., Williams D. L. Biosynthesis of the vitellogenins. Identification and characterization of nonphosphorylated precursors to avian vitellogenin I and vitellogenin II. J Biol Chem. 1982 Apr 10;257(7):3837–3846. [PubMed] [Google Scholar]
- Wibo M., Amar-Costesec A., Berthet J., Beaufay H. Electron microscope examination of subcellular fractions. 3. Quantitative analysis of the microsomal fraction isolated from rat liver. J Cell Biol. 1971 Oct;51(1):52–71. doi: 10.1083/jcb.51.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]