Abstract
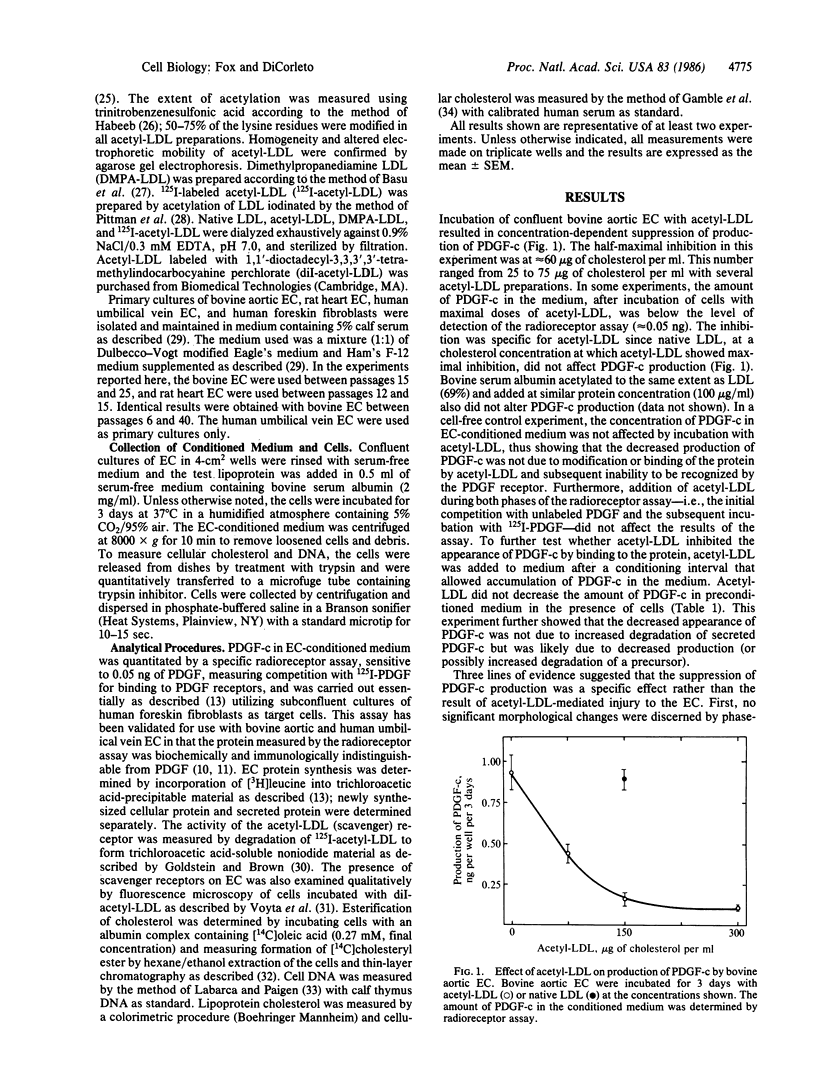
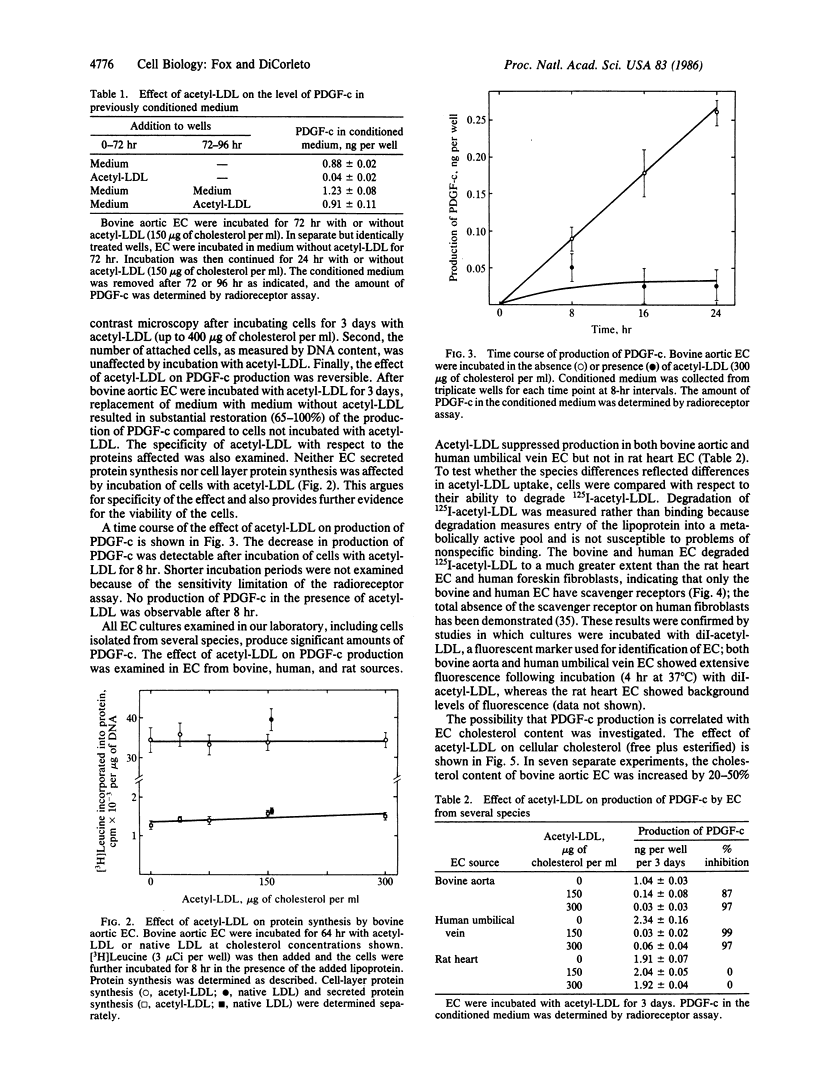
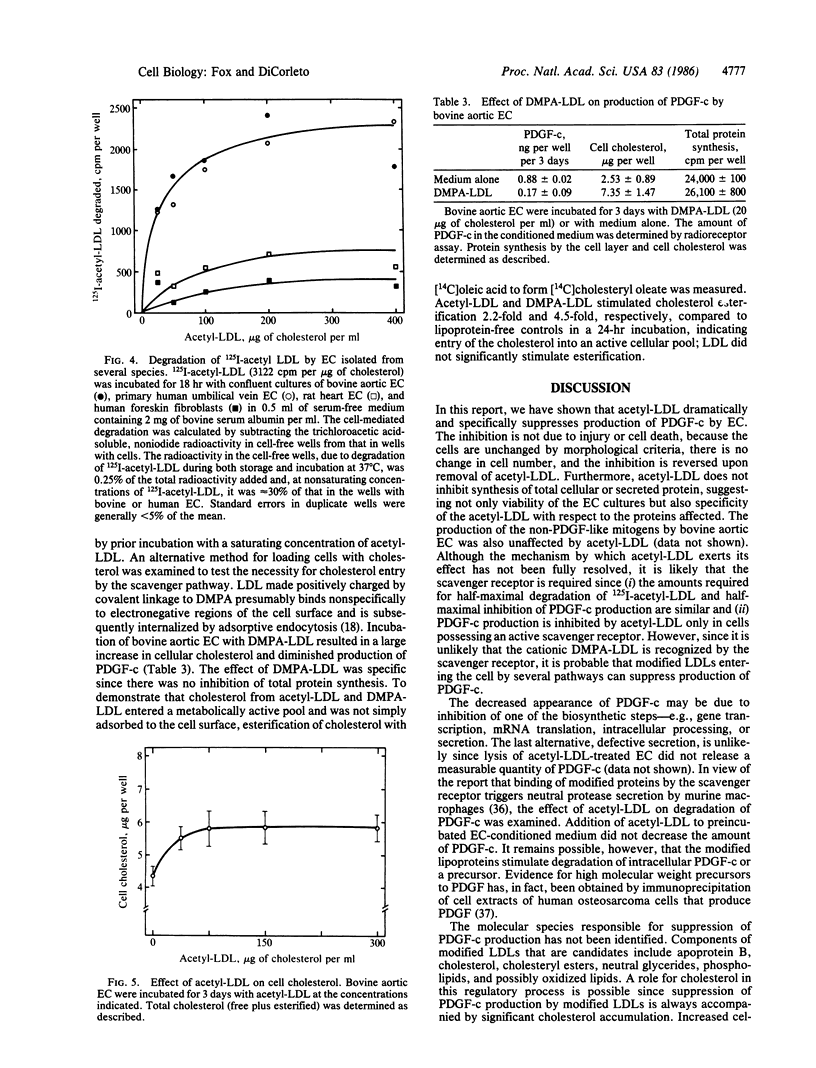
Cultured endothelial cells (EC) produce a platelet-derived growth factor-like protein (PDGF-c) that stimulates the growth of cultured cells of mesenchymal origin. We have examined the effect of native plasma low density lipoprotein (LDL) and chemically modified LDL on production of PDGF-c by EC. Acetyl-LDL, but not native LDL, suppressed the production of PDGF-c by bovine aortic EC. Half-maximal inhibition was observed at a concentration of 25-75 micrograms of cholesterol per ml, and maximal inhibition (0-25% of control) at 150 micrograms of cholesterol per ml. EC treated with acetyl-LDL showed no morphological damage, there was no change in cell number, and the effect on production of PDGF-c was substantially reversed upon removal of the acetyl-LDL. The observed inhibition of PDGF-c production was specific, since total cellular and secreted protein synthesis were unaffected by acetyl-LDL. Acetyl-LDL suppressed PDGF-c production in both bovine aortic and human umbilical vein EC, but not in rat heart EC. This cell specificity correlated with the presence of scavenger receptors as measured by degradation of 125I-labeled acetyl-LDL and uptake of fluorescently labeled acetyl-LDL. Dimethylpropanediamine-LDL, a cationic modified lipoprotein, also inhibited PDGF-c production. The inhibition by both types of modified LDL was accompanied by significant intracellular cholesterol accumulation, suggesting a role for EC lipid composition in the regulation of production of PDGF-c.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D. P., Van Lenten B. J., Fogelman A. M., Edwards P. A., Kean C., Berliner J. A. LDL, scavenger, and beta-VLDL receptors on aortic endothelial cells. Arteriosclerosis. 1984 May-Jun;4(3):248–255. doi: 10.1161/01.atv.4.3.248. [DOI] [PubMed] [Google Scholar]
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner J. A., Frank H. J., Karasic D., Capdeville M. Lipoprotein-induced insulin resistance in aortic endothelium. Diabetes. 1984 Nov;33(11):1039–1044. doi: 10.2337/diab.33.11.1039. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Brinton E. A., Kenagy R. D., Oram J. F., Bierman E. L. Regulation of high density lipoprotein binding activity of aortic endothelial cells by treatment with acetylated low density lipoprotein. Arteriosclerosis. 1985 Jul-Aug;5(4):329–335. doi: 10.1161/01.atv.5.4.329. [DOI] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E. Cultured endothelial cells produce multiple growth factors for connective tissue cells. Exp Cell Res. 1984 Jul;153(1):167–172. doi: 10.1016/0014-4827(84)90458-0. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., de la Motte C. A. Characterization of the adhesion of the human monocytic cell line U937 to cultured endothelial cells. J Clin Invest. 1985 Apr;75(4):1153–1161. doi: 10.1172/JCI111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust J. R., Luskey K. L., Chin D. J., Goldstein J. L., Brown M. S. Regulation of synthesis and degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by low density lipoprotein and 25-hydroxycholesterol in UT-1 cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5205–5209. doi: 10.1073/pnas.79.17.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. L., DiCorleto P. E. Regulation of production of a platelet-derived growth factor-like protein by cultured bovine aortic endothelial cells. J Cell Physiol. 1984 Nov;121(2):298–308. doi: 10.1002/jcp.1041210206. [DOI] [PubMed] [Google Scholar]
- Gajdusek C., DiCorleto P., Ross R., Schwartz S. M. An endothelial cell-derived growth factor. J Cell Biol. 1980 May;85(2):467–472. doi: 10.1083/jcb.85.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Progress in understanding the LDL receptor and HMG-CoA reductase, two membrane proteins that regulate the plasma cholesterol. J Lipid Res. 1984 Dec 15;25(13):1450–1461. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Hoff H. F., Ho Y. K., Basu S. K., Brown M. S. Stimulation of cholesteryl ester synthesis in macrophages by extracts of atherosclerotic human aortas and complexes of albumin/cholesteryl esters. Arteriosclerosis. 1981 May-Jun;1(3):210–226. doi: 10.1161/01.atv.1.3.210. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Owen A. J., Barth R. K., Tempst P., Winoto A., Fors L., Hood L. E., Antoniades H. N. Detection of c-sis transcripts and synthesis of PDGF-like proteins by human osteosarcoma cells. Science. 1984 Nov 23;226(4677):972–974. doi: 10.1126/science.6209798. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Platelet-derived growth factor. Mol Cell Endocrinol. 1985 Mar;39(3):169–187. doi: 10.1016/0303-7207(85)90061-9. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Morel D. W., Lewis L. J., Chisolm G. M. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983 May-Jun;3(3):215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- Jaye M., McConathy E., Drohan W., Tong B., Deuel T., Maciag T. Modulation of the sis gene transcript during endothelial cell differentiation in vitro. Science. 1985 May 17;228(4701):882–885. doi: 10.1126/science.3890179. [DOI] [PubMed] [Google Scholar]
- Johnson W. J., Pizzo S. V., Imber M. J., Adams D. O. Receptors for maleylated proteins regulate secretion of neutral proteases by murine macrophages. Science. 1982 Nov 5;218(4572):574–576. doi: 10.1126/science.6289443. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A. J., Pantazis P., Antoniades H. N. Simian sarcoma virus--transformed cells secrete a mitogen identical to platelet-derived growth factor. Science. 1984 Jul 6;225(4657):54–56. doi: 10.1126/science.6328659. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Bovine aortic endothelial cells display macrophage-like properties towards acetylated 125I-labelled low density lipoprotein. Biochim Biophys Acta. 1980 Dec 5;620(3):631–635. doi: 10.1016/0005-2760(80)90155-1. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. H., Largis E. E., Schaffer S. A. The effects of endothelial cell-conditioned media on the proliferation of aortic smooth muscle cells and 3T3 cells in culture. Artery. 1981;9(5):358–371. [PubMed] [Google Scholar]
- Witte L. D., Cornicelli J. A., Miller R. W., Goodman D. S. Effect of platelet-derived and endothelial cell-derived growth factors on the low density lipoprotein receptor pathway in cultured human fibroblasts. J Biol Chem. 1982 May 25;257(10):5392–5401. [PubMed] [Google Scholar]


