Abstract
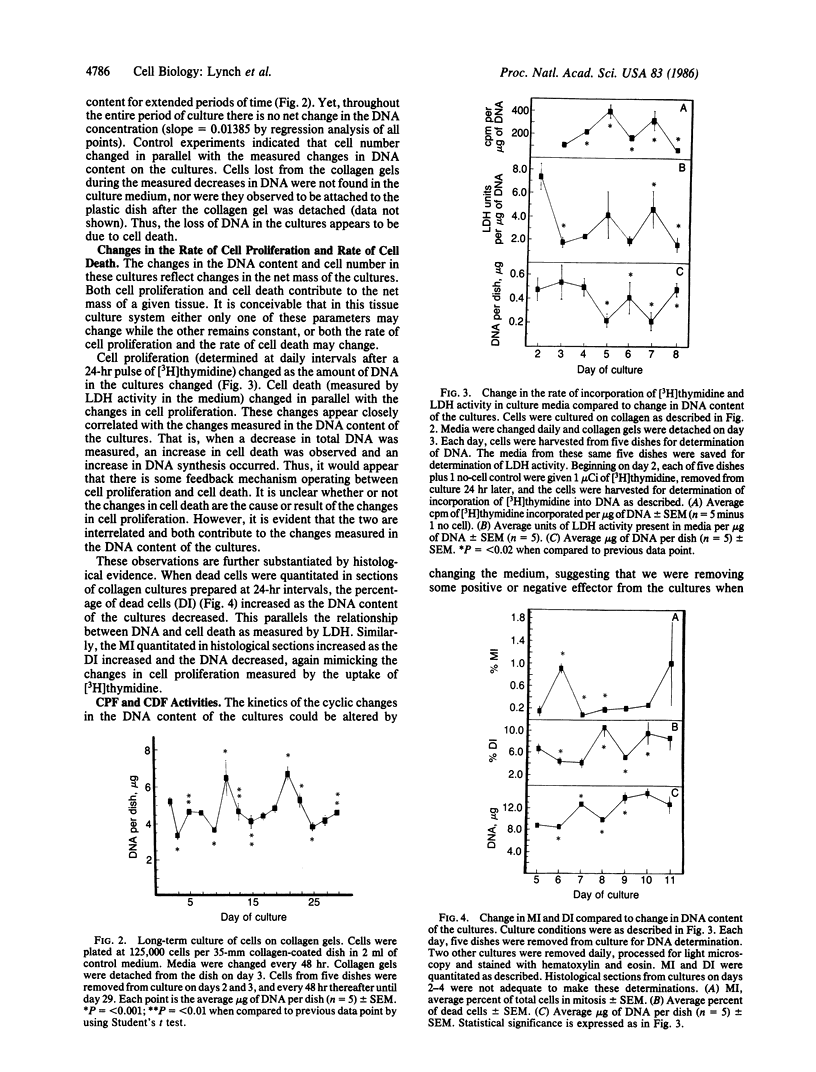
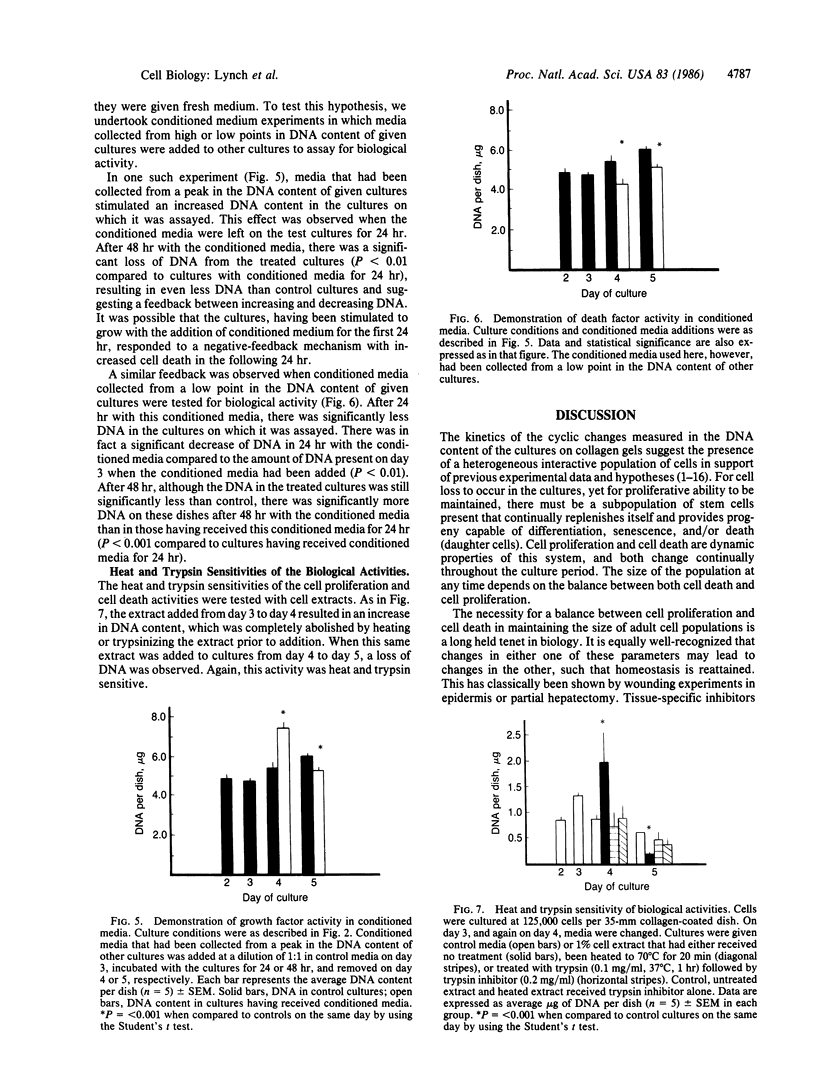
Primary cultures of rabbit endometrial cells grown on collagen substrates exhibit cyclic changes in DNA content throughout extended periods of culture. These cycles are characterized by periods of significant increases and decreases in the DNA content of the cultures or number of cells present, yet through the entire duration of culture there is no net change in the total DNA. The rates of cell proliferation and cell death change through time in culture with the same periodicity as the changes in DNA. Neither changes in the rate of cell proliferation nor the rate of cell death alone are sufficient to account for the changes in DNA. Rather, there appears to be a feedback mechanism operating between cell proliferation and cell death such that when one increases, the other increases concomitantly in order to maintain a homeostasis in total culture mass. This homeostasis appears to be mediated by a soluble cell proliferation factor (CPF) and a cell death factor (CDF) produced by the cells. CPF and CDF may be obtained from either conditioned media or cultured cell extracts. These biological activities are heat and trypsin sensitive. The major mode of cell death in these cultures appears to be apoptosis or programmed cell death, characteristic of renewing epithelia. The data suggest that this tissue culture model system represents a renewing cell population containing stem cells and their progeny, whose total growth is strictly regulated by CPF and CDF. As such, it provides a model system in which to study homeostasis and how it may be altered in hyperplasia and neoplasia, as well as its regulation by hormones.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berliner J. A., Gerschenson L. E. Sex steroid induced morphological changes in primary uterine cell cultures. J Steroid Biochem. 1976 Mar;7(3):153–158. doi: 10.1016/0022-4731(76)90196-5. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Conti C. J., Conner E. A., Gimenez-Conti I. B., Silverberg S. G., Gerschenson L. E. Regulation of ciliogenesis and proliferation of uterine epithelium by 20 alpha-hydroxy-pregn-4-en-3-one administration and withdrawal in ovariectomized rabbits. Biol Reprod. 1981 May;24(4):903–911. doi: 10.1095/biolreprod24.4.903. [DOI] [PubMed] [Google Scholar]
- Conti C. J., Gimenez-Conti I. B., Conner E. A., Lehman J. M., Gerschenson L. E. Estrogen and progesterone regulation of proliferation, migration, and loss in different target cells of rabbit uterine epithelium. Endocrinology. 1984 Feb;114(2):345–351. doi: 10.1210/endo-114-2-345. [DOI] [PubMed] [Google Scholar]
- Conti C. J., Gimenez-Conti I. B., Zerbe G. O., Gerschenson L. E. Differential effects of estradiol-17 beta and progesterone on the proliferation of glandular and luminal cells of rabbit uterine epithelium. Biol Reprod. 1981 Apr;24(3):643–648. doi: 10.1095/biolreprod24.3.643. [DOI] [PubMed] [Google Scholar]
- GREENE H. S. Adenocarcinoma of the uterine fundus in the rabbit. Ann N Y Acad Sci. 1959 Jan 9;75:535–542. doi: 10.1111/j.1749-6632.1959.tb44573.x. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Berliner J. A. Further studies on the regulation of cultured rabbit endometrial cells by diethylstilbestrol and progesterone. J Steroid Biochem. 1976 Mar;7(3):159–165. doi: 10.1016/0022-4731(76)90197-7. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Berliner J., Yang J. Diethylstilbestrol and progesterone regulation of cultured rabbit endometrial cell growth. Cancer Res. 1974 Nov;34(11):2873–2880. [PubMed] [Google Scholar]
- Gerschenson L. E., Conner E. A., Yang J., Andersson M. Hormonal regulation of proliferation in two populations of rabbit endometrial cells in culture. Life Sci. 1979 Apr 9;24(15):1337–1343. doi: 10.1016/0024-3205(79)90002-x. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Conner E., Murai J. T. Regulation of the cell cycle by diethylstilbestrol and progesterone in cultured endometrial cells. Endocrinology. 1977 May;100(5):1468–1471. doi: 10.1210/endo-100-5-1468. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Conti C. J., Depaoli J. R., Lieberman R., Lynch M., Orlicky D., Rivas-Berrios A. Estrogen and progesterone regulation of proliferation and differentiation of rabbit uterine epithelium. Prog Clin Biol Res. 1984;142:119–132. [PubMed] [Google Scholar]
- Gerschenson L. E., Depaoli J. R., Murai J. T. Inhibition of estrogen-induced proliferation of cultured rabbit uterine epithelial cells by a cell density-dependent factor produced by the same cells. J Steroid Biochem. 1981 Oct;14(10):959–969. doi: 10.1016/0022-4731(81)90203-x. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Fennell R. H., Jr A developmental view of endometrial hyperplasia and carcinoma based on experimental research. Pathol Res Pract. 1982 Aug;174(3):285–296. doi: 10.1016/S0344-0338(82)80071-X. [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Sulston J. E., Thomson J. N. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science. 1983 Jun 17;220(4603):1277–1279. doi: 10.1126/science.6857247. [DOI] [PubMed] [Google Scholar]
- Hopwood D., Levison D. A. Atrophy and apoptosis in the cyclical human endometrium. J Pathol. 1976 Jul;119(3):159–166. doi: 10.1002/path.1711190305. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R., Sternberg P. W., Greenwald I. S., Fixsen W., Ellis H. M. Mutations that affect neural cell lineages and cell fates during the development of the nematode Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):453–463. doi: 10.1101/sqb.1983.048.01.050. [DOI] [PubMed] [Google Scholar]
- Kistner R. W., Griffiths C. T., Craig J. M. Use of progestational agents in the management of endometrial cancer. Cancer. 1965 Dec;18(12):1563–1579. doi: 10.1002/1097-0142(196512)18:12<1563::aid-cncr2820181208>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Murai J. T., Conner E., Yang J., Andersson M., Berliner J., Gerschenson L. E. Hormonal regulation of cultured rabbit endometrial cells. J Toxicol Environ Health. 1978 Mar-May;4(2-3):449–456. doi: 10.1080/15287397809529670. [DOI] [PubMed] [Google Scholar]
- Murai J. T., Conti C. J., Gimenez-Conti I., Orlicky D., Gerschenson L. E. Temporal relationship between rabbit uterine epithelium proliferation and uteroglobin production. Biol Reprod. 1981 Apr;24(3):649–653. doi: 10.1095/biolreprod24.3.649. [DOI] [PubMed] [Google Scholar]
- Rajkumar K., Bigsby R., Lieberman R., Gerschenson L. E. Effect of progesterone and 17 beta-estradiol on the production of uteroglobin by cultured rabbit uterine epithelial cells. Endocrinology. 1983 Apr;112(4):1499–1505. doi: 10.1210/endo-112-4-1499. [DOI] [PubMed] [Google Scholar]
- Rajkumar K., Bigsby R., Lieberman R., Gerschenson L. E. Uteroglobin production by cultured rabbit uterine epithelial cells. Endocrinology. 1983 Apr;112(4):1490–1498. doi: 10.1210/endo-112-4-1490. [DOI] [PubMed] [Google Scholar]
- Sandow B. A., West N. B., Norman R. L., Brenner R. M. Hormonal control of apoptosis in hamster uterine luminal epithelium. Am J Anat. 1979 Sep;156(1):15–35. doi: 10.1002/aja.1001560103. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K. Steroid hormones and endometrial cancer. Cancer Res. 1978 Nov;38(11 Pt 2):4360–4366. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. The biology of cell death in tumours. Anticancer Res. 1985 Jan-Feb;5(1):131–136. [PubMed] [Google Scholar]