Abstract
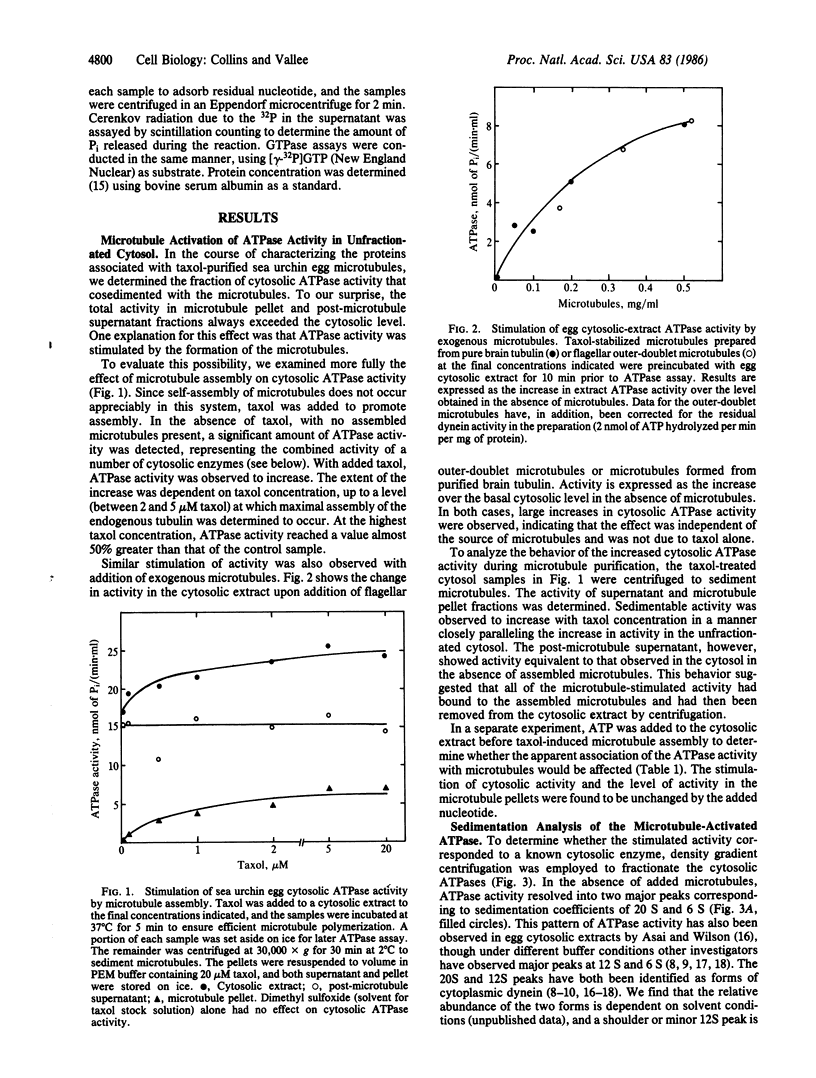
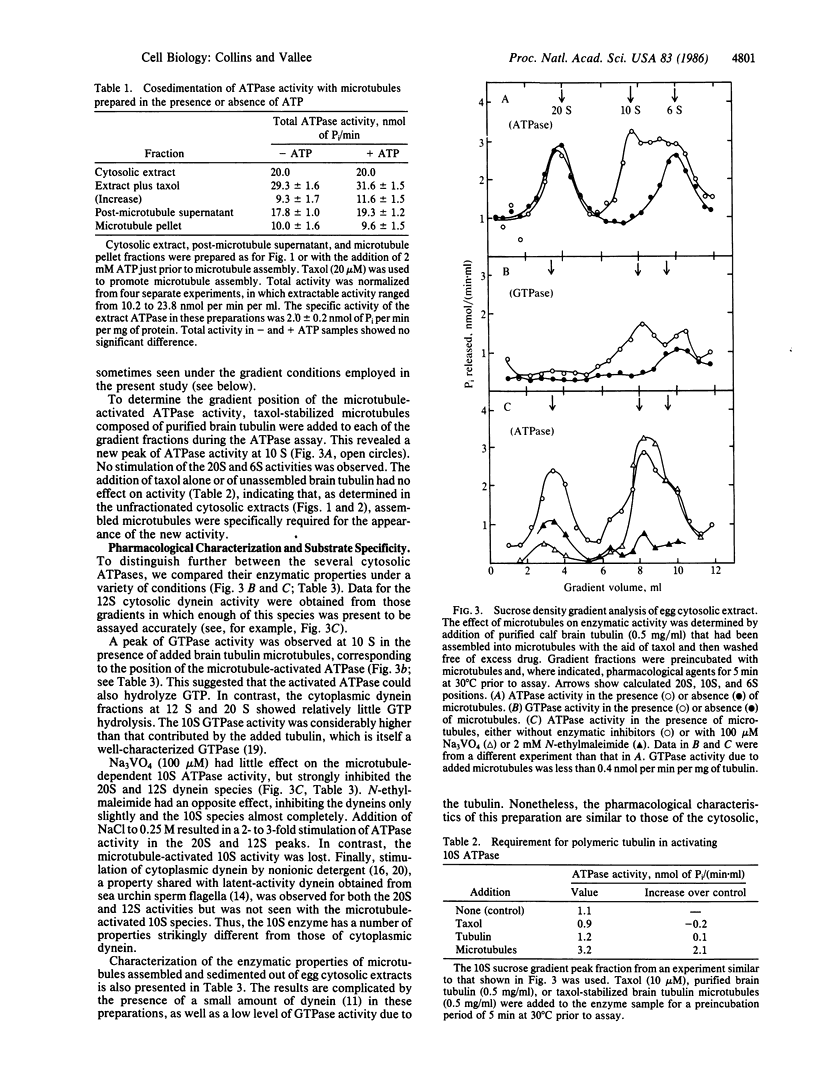
We report an ATPase activity, present in sea urchin egg cytosol, that is activated by microtubules. The activity sediments at 10 S in sucrose gradients and is clearly distinct from activities at 12 S and 20 S due to cytoplasmic dynein. Potent activation of the ATPase is observed when endogenous egg tubulin is induced to assemble with taxol or when exogenous taxol-stabilized pure brain tubulin microtubules or flagellar outer-doublet microtubules are added. No activation by tubulin subunits or taxol alone is detectable. In contrast to flagellar or cytoplasmic dynein, the microtubule-activated enzyme is unaffected by vanadate or by nonionic detergents and hydrolyzes GTP in addition to ATP. In contrast to kinesin, it cosediments with microtubules in the presence or absence of ATP. The microtubule-activated enzyme may have a role in microtubule-based motility.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai D. J., Wilson L. A latent activity dynein-like cytoplasmic magnesium adenosine triphosphatase. J Biol Chem. 1985 Jan 25;260(2):699–702. [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Collins C. A., Vallee R. B. Use of multiple monoclonal antibodies to characterize the major microtubule-associated protein in sea urchin eggs. Cell Motil. 1985;5(6):431–446. doi: 10.1002/cm.970050602. [DOI] [PubMed] [Google Scholar]
- Brady S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985 Sep 5;317(6032):73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Burns R. G., Pollard T. D. A dynein-like protein from brain. FEBS Lett. 1974 Apr 1;40(2):274–280. doi: 10.1016/0014-5793(74)80243-7. [DOI] [PubMed] [Google Scholar]
- Cande W. Z. Nucleotide requirements for anaphase chromosome movements in permeabilized mitotic cells: anaphase B but not anaphase A requires ATP. Cell. 1982 Jan;28(1):15–22. doi: 10.1016/0092-8674(82)90370-1. [DOI] [PubMed] [Google Scholar]
- Cande W. Z., Wolniak S. M. Chromosome movement in lysed mitotic cells is inhibited by vanadate. J Cell Biol. 1978 Nov;79(2 Pt 1):573–580. doi: 10.1083/jcb.79.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Kondo Y., Kumasaka M., Suzuki T., Ohki K. Stimulation of tubulin-dependent ATPase activity in microtubule proteins from porcine brain by taxol. J Neurochem. 1983 Sep;41(3):716–722. doi: 10.1111/j.1471-4159.1983.tb04799.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Simultaneous localization of myosin and tubulin in human tissue culture cells by double antibody staining. J Cell Biol. 1978 Apr;77(1):182–195. doi: 10.1083/jcb.77.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand V. I., Gyoeva F. K., Rosenblat V. A., Shanina N. A. A new ATPase in cytoplasmic microtubule preparations. FEBS Lett. 1978 Apr 15;88(2):197–200. doi: 10.1016/0014-5793(78)80172-0. [DOI] [PubMed] [Google Scholar]
- Gibbons I. R., Fronk E. A latent adenosine triphosphatase form of dynein 1 from sea urchin sperm flagella. J Biol Chem. 1979 Jan 10;254(1):187–196. [PubMed] [Google Scholar]
- Hisanaga S., Pratt M. M. Calmodulin interaction with cytoplasmic and flagellar dynein: calcium-dependent binding and stimulation of adenosinetriphosphatase activity. Biochemistry. 1984 Jun 19;23(13):3032–3037. doi: 10.1021/bi00308a029. [DOI] [PubMed] [Google Scholar]
- Hisanaga S., Sakai H. Cytoplasmic dynein of the sea urchin egg. II. Purification, characterization and interactions with microtubules and Ca-calmodulin. J Biochem. 1983 Jan;93(1):87–98. doi: 10.1093/oxfordjournals.jbchem.a134182. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Fujii T., Arai T., Tanaka R., Kaziro Y. The presence of an adenosine-5'-triphosphatase dependent on 6S tubulin and calcium ions in rat brain microtubules. J Biochem. 1979 Aug;86(2):587–590. doi: 10.1093/oxfordjournals.jbchem.a132560. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Smith H., Taylor E. W. Tublin: nucleotide binding and enzymic activity. J Mol Biol. 1974 Nov 5;89(3):455–468. doi: 10.1016/0022-2836(74)90475-6. [DOI] [PubMed] [Google Scholar]
- Kiehart D. P., Mabuchi I., Inoué S. Evidence that myosin does not contribute to force production in chromosome movement. J Cell Biol. 1982 Jul;94(1):165–178. doi: 10.1083/jcb.94.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Martensen T., Nath J., Flavin M. Inhibition of dynein ATPase by vanadate, and its possible use as a probe for the role of dynein in cytoplasmic motility. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1313–1318. doi: 10.1016/0006-291x(78)91279-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Ogawa K., Mohri H. Evidence that the Mg-ATPase in the cortical layer of sea urchin egg is dynein. Exp Cell Res. 1978 Jul;114(2):285–292. doi: 10.1016/0014-4827(78)90485-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linck R. W., Langevin G. L. Reassembly of flagellar B (alpha beta) tubulin into singlet microtubules: consequences for cytoplasmic microtubule structure and assembly. J Cell Biol. 1981 May;89(2):323–337. doi: 10.1083/jcb.89.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Hiebsch R. R., Wallis K. T. Identity and Origin of the ATPase activity associated with neuronal microtubules. I. The ATPase activity is associated with membrane vesicles. J Cell Biol. 1983 May;96(5):1298–1305. doi: 10.1083/jcb.96.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T., Hiebsch R. R. Identity and origin of the ATPase activity associated with neuronal microtubules. II. Identification of a 50,000-dalton polypeptide with ATPase activity similar to F-1 ATPase from mitochondria. J Cell Biol. 1983 May;96(5):1306–1315. doi: 10.1083/jcb.96.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallini V., Mencarelli C., Bracci L., Contorni M., Ruggiero P., Tiezzi A., Manetti R. Cytoplasmic nucleoside-triphosphatase similar to axonemal dynein occur widely in different cell types. J Submicrosc Cytol. 1983 Jan;15(1):229–235. [PubMed] [Google Scholar]
- Pratt M. M., Otter T., Salmon E. D. Dynein-like Mg2+-ATPase in mitotic spindles isolated from sea urchin embryos (Strongylocentrotus droebachiensis). J Cell Biol. 1980 Sep;86(3):738–745. doi: 10.1083/jcb.86.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt M. M. The identification of a dynein ATPase in unfertilized sea urchin eggs. Dev Biol. 1980 Feb;74(2):364–378. doi: 10.1016/0012-1606(80)90438-8. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Neighbors B., McIntosh J. R., Salmon E. D. Isolation of microtubules and a dynein-like MgATPase from unfertilized sea urchin eggs. J Biol Chem. 1984 May 25;259(10):6516–6525. [PubMed] [Google Scholar]
- Scholey J. M., Porter M. E., Grissom P. M., McIntosh J. R. Identification of kinesin in sea urchin eggs, and evidence for its localization in the mitotic spindle. Nature. 1985 Dec 5;318(6045):483–486. doi: 10.1038/318483a0. [DOI] [PubMed] [Google Scholar]
- Tominaga S., Hirosawa K., Kaziro Y. Presence of two distinct adenosine triphosphatase activities in bovine brain microtubules. FEBS Lett. 1982 Jul 19;144(1):112–116. doi: 10.1016/0014-5793(82)80581-4. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Bloom G. S. Isolation of sea urchin egg microtubules with taxol and identification of mitotic spindle microtubule-associated proteins with monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6259–6263. doi: 10.1073/pnas.80.20.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Borisy G. G. The non-tubulin component of microtubule protein oligomers. Effect on self-association and hydrodynamic properties. J Biol Chem. 1978 Apr 25;253(8):2834–2845. [PubMed] [Google Scholar]
- White H. D., Coughlin B. A., Purich D. L. Adenosine triphosphatase activity of bovine brain microtubule protein. J Biol Chem. 1980 Jan 25;255(2):486–491. [PubMed] [Google Scholar]



