Abstract
Metastatic melanoma remains one of the most lethal and poorly treated forms of human cancer. Its incidence is on the rise, but no therapies offering improved survival rates have been developed over the last 40 years. This has changed with the recent Food and Drug Administration (FDA) approval of the CTLA-4 function blocking antibody Ipilimumab (Yervoy), proven to extend life in patients with previously treated or untreated metastatic melanoma [39,40]. CTLA-4 is a receptor that normally functions to inhibit inappropriate or prolonged activation of T-cells. This review presents the history of initial research into the function of the CTLA-4 receptor, the pre-clinical evidence for CTLA-4 blockade’s utility in cancer treatment, and the recent human clinical trials that have proven its efficacy in advanced stage melanoma. Ipilimumab represents one of a growing class of cancer immunotherapies currently under development and highlights both the promise and relative infancy of these agents in the clinical setting.
Keywords: melanoma, cancer, ipilimumab, CTLA-4, T-cells, dermatology, skin, immunotherapy, immunology
Introduction
Metastatic melanoma has been described as one of the most aggressive forms of human cancer, and its incidence is on the rise. It originates from uncontrolled proliferation of specialized melanocytes normally responsible for producing pigments in epithelial layers. Though typically associated with the skin, these cells are also present in the eye, ears, meninges, bone, and heart, and cancer lesions can develop in any of these locations.
In 2010, nearly 70,000 Americans were diagnosed with either invasive or in situ melanoma, proving fatal in about 9,000 cases annually; 1 in 39 Caucasian males (about 2.5 percent) born in 2010 are expected to develop melanoma in their lifetime [1]. This rate stood at around 1 in 1,500 (or less than .06 percent) in 1935, indicating the dramatic increase in prevalence of melanoma over the last century [2].
Despite being a rare form of skin cancer, melanoma accounts for nearly 75 percent of skin cancer deaths. While those with early Stage I lesions have high 3-year survival rates (more than 90 percent), individuals with late-stage melanoma have a poorer prognosis (10 percent) with a median survival of only 7.5 months after diagnosis [3]. For the last 40 years, treatment advances have been largely stagnant. Traditional options for late-stage patients lack substantial efficacy. These include IL-2, which shows only a 6 percent complete response rate [4], and dacarbazine, which produces only a 6 percent to 15 percent response rate with no improvement in survival [5].
However, the landscape for late-stage treatment options has changed recently with the FDA approval in March 2011 of the cancer immunotherapy drug ipilimumab for treatment of metastatic melanoma [6]. Widely touted as a therapeutic breakthrough, ipilimumab works through enhancing T-cell activity by modifying the function of the Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) inhibitory receptor. Evidence for ipilimumab offers hope for patients with a clearly lethal disease, but also highlights some of the dangers and relative infancy of immunotherapies in the clinical setting.
CTLA-4 AND CANCER IMMUNOTHERAPY
While many chemotherapy drugs target cancer cells through manipulation of the cell cycle and apoptosis, immunotherapy alternatively relies on augmentation of the immune mechanisms responsible for naturally eliminating cancer cells. This approach is not necessarily “new,” as researchers have been developing these agents for more than a century alongside an explosion in our knowledge of the human immune system. As early as 1890, Paul Ehrlich proposed the use of active immunization as a treatment for cancer [7].
Nearly 70 years later, Sir Frank Burnet of Australia hypothesized the concept of cancer surveillance: the ability of the immune system to recognize and eliminate transformed cells [8]. By the early 1990s, Rituximab was released as the first antibody-based therapy for a human cancer, B-cell lymphoma [9]. Other therapies followed, including IL-2 [10] and interferon-alpha treatment [11,12]. The field is expanding, and immunotherapies ranging from adoptive T-cell transfer for renal cancer [13] to radioconjugated antibodies for malignant gliomas [14] are now being tested in the clinic.
Recent studies have shown that melanoma lesions often contain a high number of infiltrative T-cells specific to melanocyte tumor associated antigens such as MART1, gp-100, and tyrosinase, which are components of the melanin synthesis pathway [15]. Augmenting the natural function of these Cytotoxic T-Lymphocytes (CTLs) seems a logical approach to eliminating melanoma cancer cells. Normal T-cell activation requires two complementary signals. This includes TCR stimulation by MHC bound antigens and by interaction of the T-cell’s CD28 receptor with the B7 receptor (CD80/CD86) found on APCs [16]. Stimulation leads to intracellular signaling activity that helps initiate T-cell activation, promoting the release of IL-2 and enhancing proliferation.
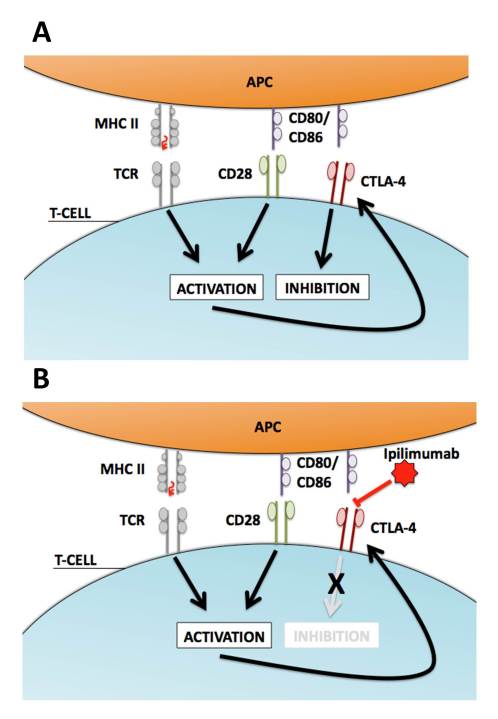
CTLA-4 was discovered in the late 1980s and first identified as an immunoglobin found primarily on CD4+ or CD8+ T-lymphocytes with a then-unidentified function [17]. Other studies have demonstrated that high levels of CTLA-4 are also important in maintaining certain subsets of T-regulatory cells [18]. CTLA-4, like CD28, binds B7 receptors on APCs. It alternatively initiates inhibitory effects upon binding, including cell cycle arrest and decreased cytokine production. More importantly, some forms of the B7 receptor show dramatically increased binding affinity for CTLA-4 over CD28 [19], as a single CTLA-4 receptor can concurrently bind as many as eight B7 molecules [20]. A balancing act thus occurs between CD28/B7 mediated activating signals and CTLA-4/B7 inhibitory signals in normal T-cells to help initiate robust killing but also to serve to prevent prolonged activation when inappropriate (Figure 1A).
Figure 1.
T-cell regulation, CTLA-4, and Ipilimumab. A) Normal T-cell activation occurs via presentation of MHC-bound antigens on APCs that signal through the TCR. This process is amplified by the co-stimulatory signal produced by interaction of the CD80/86 (B7) receptor on APCs with CD28 molecules on the T-cell. Prolonged T-cell activation initiates the upregulation of CTLA-4, a membrane receptor that competes with CD28 for binding to CD80/86 (B7). CTLA-4 has a higher affinity for CD80/86 (B7) than CD28 and its binding oppositely produces inhibitory signals through decreased proliferation and IL-2 production. This relationship allows normal T-cells to prevent over-activation through feedback upregulation of CTLA-4. B) Ipilimumab binds to CTLA-4 and prevents interaction with CD80/CD86 (B7) molecules on APCs. This removes CTLA-4 induced inhibitory signals allowing for unregulated and prolonged activation of T-cells in a non-specific manner.
PRE-CLINICAL EVIDENCE FOR THE DEVELOPMENT OF IPILIMUMAB
CTLA-4 became an attractive target for immunotherapy once it was identified as a negative regulator of T-cell activation. Pre-clinical evidence began to provide strong support that CTLA-4 modification could serve as a useful approach for controlling natural immune responses in animal models of common human malignancies. Researchers first constructed functional blocking and enhancing monoclonal antibodies against murine CTLA-4. Blockade functions in theory to remove CTLA-4 inhibitory signals such that T-cell co-activation via B7/CD28 interactions becomes unopposed, leading to increased T-cell activity (Figure 1B).
Walunas et al. demonstrated that CTLA-4 blockade helped augment T-cell proliferation and activation in vivo using targeted antibody treatment [21]. In a complete knockout murine model, CTLA-4 loss results in massive lymphoproliferation, organ destruction, and death [22,23], a finding that predicted some of the many risks associated with CTLA-4 blockade in humans discussed later in this review. Krummel and Allison alternatively used activating antibodies and showed that CTLA-4 activation blocked T-cell proliferation and decreased IL-2 production [24]. It is with these initial studies that scientists proved capable of modulating CTLA-4 activity in T-cells in vivo using targeted antibodies.
Researchers next hypothesized that CTLA-4 modulation might produce clinically useful results in tumor models. Many human cancers contain T-cells that are not properly activated against target cells expressing tumor-associated antigens (TAAs), including melanoma. It is generally thought that these TAAs are unable to initiate sufficient activating signals via the B7/CD28 and MHC/TCR co-stimulatory pathways when opposed by CTLA-4 [25]. Evidence shows that T-cells isolated from mice transplanted with a fibrosarcoma decrease their capacity to produce lymphocytokines over the course of a few weeks. This is rescued by CTLA-4 blockade, which increased levels of IL-2 and INF-gamma production in a tumor stage dependent manner [26]. Accordingly, a number of studies were conducted to test if CTLA-4 blockade could be used to treat common human tumors in pre-clinical animal models.
CTLA-4 blockade was first tested across a number of murine models of cancer, including prostate cancer [27], breast cancer [28], and lymphoma [29]. Researchers often found that the combination of CTLA-4 therapy with a vaccine or some other immune stimulating factor proved most efficacious. For instance, using a prostate cancer mouse model [30], researchers were able to reduce tumor incidence five-fold with a combined treatment of a CTLA-4 antibody and an irradiated tumor vaccine [31].
For melanoma, the approach to combine CTLA-4 blockade with another form of immune modulation was also considered. In one study, mice transplanted with a poorly immunogenic melanoma cell line showed up to an 80 percent cure rate in recently injected tumors when CTLA-4 antibody blockade was combined with a GM-CSF vaccine [32]. Other pre-clinical research demonstrated that CTLA-4 inhibition paired with DNA vaccines targeted against gp100 or tyrosinase-2 worked synergistically to improve tumor eradication [33]. Ultimately, these works showed that augmentation of naturally present CTLs in cancer tissues through CTLA-4 blockade could provide clinical benefit in animal models of melanoma and other human malignancies.
THE DEVELOPMENT OF IPILUMUMAB AND CLINICAL EVIDENCE IN ADVANCED STAGE MELANOMA
Elucidation of the basic role of CTLA-4 in immune modulation and the success of pre-clinical studies in murine models of cancer provided strong impetus for the creation of a human CTLA-4 antibody. This set the path for the development of ipilimumab (Yervoy), which originated in the lab of Dr. James Allison, formerly of the University of California-Berkeley. His team was one of the first to identity and describe the role of CTLA-4 in immune function. The lab also developed a number of antibody-based approaches for blockade [34]. These CTLA-4 antibody technologies were acquired by a private biotechnology startup, Medarex, through a patent acquisition in 2000.
Medarex began recruiting for a Phase III trial in 2004 following initial data from earlier Phase I/II studies indicating ipilimumab was safe and potentially efficacious for treatment of late stage melanoma [35,36]. By 2009, Medarex became a subsidiary of pharmaceutical giant Bristol-Meyers Squibb in order to further commercialize ipilimumab for use in melanoma and other cancers. Pfizer was also concurrently developing its own CTLA-4 antibody, tremelimumab, which was later abandoned based off poor Phase III results [37]. Alternatively, ipilimumab was granted permission to be marketed to treat advanced melanoma patients in the European Union in 2010 [38] and more recently approved by the FDA for treatment of metastatic melanoma in the United States.
This decision culminated a substantial development process that has run parallel with a number of clinical studies for the use of ipilimumab in many other human cancers (Table 1). The Phase III studies that supported these advances resulted in two high profile articles, both published in the New England Journal of Medicine. Researchers demonstrated that ipilimumab treatment increased survival rates in patients both with previously treated and untreated metastatic melanoma.
Table 1. History of the Development of Ipilimumab.
| Month | Year | Development |
| January | 2000 | Medarex acquires anti-CLA-4 monoclonal antibody license in the US |
| November | 2001 | Phase I/II clinical trials for malignant melanoma and prostate cancer begin in US |
| September | 2002 | Phase II clinical trials for lymphoma begin in US |
| June | 2003 | Phase I/II clinical trials for breast cancer begin in US |
| April | 2004 | Phase II trial for renal cancer begins in US |
| June | 2004 | Ipilimumab receives Orphan Drug Status for malignant melanoma in the US |
| July | 2004 | FDA approval for a Phase III study of ipilimumab for malignant melanoma in the US |
| September | 2004 | Phase III trials for melanoma begin in the US |
| November | 2004 | Ipilimumab is licensed to Bristol-Myers Squibb outside of the US |
| March | 2005 | Phase II trias for breast cancer begin globally |
| November | 2006 | Ipilimumab receive fast-track status as a second-line treatment and as a first-line treatment in combination with dacarbazine for malignant melanoma in the US |
| January | 2007 | Medarex completes enrollment of single arm of a Phase III trial for malignant melanoma |
| October | 2007 | Phase I trial for urogenital cancer begins in US |
| April | 2008 | Phase II trial for non-small cell lung cancer begins in EU and US |
| May | 2009 | Phase III trials for prostate cancer begin in Australia, Canada, the EU, Latin America, and the US |
| September | 2009 | Bristol-Myers Squibb acquires Medarex for $2.1 billion |
| August | 2010 | Phase III data published in NEJM supporting improved survival in patients with previously treated metastatic melanoma |
| August | 2010 | FDA sets decision deadline for December for ipilimumab |
| November | 2010 | FDA postpones decision until March 2011 |
| March 22 | 2011 | Bristol-Myers Squibb releases Phase III data that shows ipilimumab improves 1-year survival rates in patients with previously untreated metastatic melanoma |
| March 25 | 2011 | FDA approves ipilimumab for treatment of malignant melanoma |
| June 5 | 2011 | Phase III data officially published in NEJM supporting improved survival in patients with previously untreated metastatic melanoma |
Timeline of the development of ipilimumab along with concurrent developments for its use in other human cancers. Particular important milestones in the development of ipilimumab relevant to melanoma are noted in bold. Data adapted from figures and information presented in ipilimumab 2011 [38].
The first trial involved a randomized, double-blind study across 125 medical centers, using 676 patients with Stage III/IV melanoma who experienced disease progression after standard treatment. Patients were assigned to receive ipilimumab, a gp100 peptide vaccine, or both in combination. While patients receiving peptide vaccine alone showed a median overall survival time of 6.4 months, those receiving ipilimumab alone or in combination with the gp100 vaccine increased survival to 10.1 months and 10.0 months respectively. Responses were limited to a small subset of patients. In fact, only about 1 percent of patients showed a complete response and 5 percent to 10 percent a partial response to some form of ipilimumab treatment [39]. The release of this publication prompted the FDA to review ipilimumab as a potential therapy for late stage melanoma.
After a series of delays, the FDA approved ipilimumab for treatment of metastatic melanoma in March 2011, coinciding with the release of data from a second Phase III study. In a 502-patient, double-blind, placebo-controlled trial, Bristol-Myers Squibb compared the standard of care treatment (dacarbazine alone) with a combination treatment (ipilimumab + dacarbazine) for patients with metastatic melanoma not previously treated. Overall survival increased from 9.1 months to 11.2 months with the addition of ipilimumab, and 3-year survival increased from 12.2 percent to 20.8 percent. However, serious side effects were noted. About 56 percent of patients receiving combination therapy reported Grade 3 or 4 adverse events [40], compared to 27.5 percent of patients receiving dacarbazine alone.
IMMUNOLOGICAL CONSIDERATIONS AND RISKS OF CTLA-4 BLOCKADE
While T-cells targeting melanoma antigens are activated following treatment of ipilimumab, CTLA-4 blockade also non-specifically increases activity of all T-cells reliant upon CTLA-4 inhibition. For the case of ipilimumab, this may be particularly relevant as it is given at doses high enough that complete receptor saturation is likely [41]. Consequentially, CTLA-4 blockade can lead to immune-related adverse events that can be quite serious and are a strong criticism cited by opponents of the antibody. This has prompted some authors to compare the risk of ipilimumab to that of the recently tested CD28 agonist TGN1412 [41]. Under consideration in the United Kingdom, treatment with TGN1412 initiated a cytokine storm in some patients and caused six deaths in London during a disastrous clinical trial [42].
In both NEJM articles, nearly all patients suffered side effects from the treatment. Most commonly, these events were immune related but included diarrhea, nausea, constipation, abdominal pain, vomiting, vitiligo, and dermatitis. In the 2010 gp100 combination therapy trial, 14 deaths were attributed to be a result of taking ipilimumab, about half of which were related to severe immune events [39]. However, no deaths were reported as a result of treatment in the second Phase III trial [40].
Adverse events are a long-standing concern with ipilimumab. Case reports reveal a number of more serious consequences ranging from severe hepatitis [43] to enterocolitis [44]. In previous studies, ipilimumab has also induced hypophysitis, pancreatitis, and nephritis [45]. Of particular note, these events were more common in patients with the best tumor responses to treatment [46,47]. Consistent immune monitoring thus becomes an essential complement to ipilimumab administration. This includes monitoring cytokine levels, specific immune responses, cell population levels, and cell surface markers both in the tumor and peripherally to assess adverse events and change treatment plans and doses accordingly [48]. These immune considerations remain a major obstacle to cancer immunotherapy acceptance and highlight a challenge in targeting these agents specifically to tumor-associated immune cells.
CONCLUSIONS AND THE FUTURE OF MOLECULAR THERAPIES
Ipilimumab represents a significant advance for the treatment of metastatic melanoma and for the field of cancer immunotherapy. Originating from the discovery of a novel, immune protein in an academic laboratory, it also serves as a model example of the importance of basic science research in driving new therapeutic avenues of investigation. While ipilimumab provides hope for patients with metastatic melanoma, it is not without shortcomings.
Ultimately, most critics take the stance that cancer immunotherapies walk a fine and dangerous line between increasing natural immune responses and risking severe autoimmunity [49]. This is apparent in the widespread adverse events associated with ipilimumab treatment. Development of better immune system monitoring, markers for abnormal immune behavior, and clearer endpoints may be the best methods to overcome these challenges [48]. The clinical benefit of ipilimumab also remains relatively mild. Complete and partial responses were restricted to only a small subset of patients (about 10 percent), and ipilimumab extended the lives of all late-stage melanoma patients only by an average of a few months. Given the dangerous side effects, a better understanding of the factors that predict response levels to ipilimumab would serve to select patients best suited to receive treatment.
Despite these shortfalls, ipilimumab is a strong figure in the growing field of cancer immunotherapy. It is also not alone. New treatments for melanoma are still on the horizon. Early data suggests a novel BRAF inhibitor (vemurafenib) produces near 50 percent response rates in metastatic melanoma patients [50]. A triple peptide vaccine targeted against gp100, tyrosinase, and MART-1 has also showed promising results in increasing T-cell activity in metastatic melanoma patients [51]. More extreme approaches have utilized adoptive cell therapy to activate and expand melanoma-specific T-cells in vitro and transplant them back into patients with up to 72 percent response rates in early studies [52].
Cancer immunotherapies are novel, and their use in the clinic is still in its infancy. As we learn to enhance the efficacy of treatments like ipilimumab through better screening, immune monitoring, and continued clinical research, these innovative approaches will likely prove invaluable tools against some of the most difficult human diseases. Melanoma should likely be no exception.
Glossary
- FDA
Food and Drug Administration
- CTLA-4
Cytotoxic T-Lymphocyte Antigen-4
- APC
Antigen Presenting Cell
- IL-2
Interleukin-2
- MART-1
Melanin Antigen Recognized by T-Cells-1
- Gp-100
glycoprotein-100
- TCR
T-cell Receptor
- CTL
Cytotoxic T-Lymphocyte
- MHC
Major Histocompatibility Complex
- GM-CSF
Granulocyte-Macrophage Colony Stimulating Factor
- INF-Gamma
Interferon Gamma
- BRAF
B-Rapidly Accelerated Fibrosarcoma
References
- Jemal A. et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Rigel D. et al. The Evolution of Melanoma Diagnosis: 25 Years Beyond the ABCDs. CA Cancer J Clin. 2010;60:301–316. doi: 10.3322/caac.20074. [DOI] [PubMed] [Google Scholar]
- Laechiewicz AM. et al. Epidemiologic support for melanoma heterogeneity using the surveillance, epidemiology, and end results program. J Invest Dermatology. 2008;128(5):1340–1342. doi: 10.1038/jid.2008.18. [DOI] [PubMed] [Google Scholar]
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: Long-term survival update. Cancer Journal from Scientific American. 2000;6(Suppl 1):S11–S14. [PubMed] [Google Scholar]
- Quirbt I, Verma S, Petrella T, Bak K, Charette M. Temozolomide for the treatment of metastatic melanoma. Current Oncology. 2007;14(1):27–33. doi: 10.3747/co.2007.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor K. Ipilimumab approved for metastatic melanoma. Am J Health Syst Pharm. 2011;68(9):768. doi: 10.2146/news110025. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. In: The Collected Papers of Paul Ehrlich, Vol. II: Immunology and Cancer Research. Himmelweir B, editor. London: Pergammon; 1956. On immunity with special reference to cell life: Croonian lecture; pp. 148–192. [Google Scholar]
- Burnet FM. Cancer—a biological approach. BMJ. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P, Grillo-López AJ, Link BK. et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- Nagata S. et al. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980;284(5754):316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Waldmann T. Immunotherapy: Past, Present, and Future. Nat Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka Y, Yagi J. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60(8):1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva P, Franceschi G, Frattarelli M. et al. Loco-regional radioimmunotherapy of high-grade malignant gliomas using specific monoclonal antibodieslabeled with 90Y: a phase I study. Clin Cancer Res. 2011;5(10 Suppl):3275s–3280s. [PubMed] [Google Scholar]
- Tjin EP, Konihnenberg D, Krebbers G. et al. T cell immune function in tumor, skin, and peripheral blood of advance stage melanoma patients: implications for immunotherapy. Clin Cancer Res. 2011;17(17):5736–5747. doi: 10.1158/1078-0432.CCR-11-0230. [DOI] [PubMed] [Google Scholar]
- Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;29(1):5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet JF, Denizot F, Luciana MF. et al. A new member of the immunoglobin superfamily – CTLA-4. Nature. 1987;328(6127):267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Roncarolo MG. et al. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193(11):1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AV, Brodie DW. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17(2):201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM. et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Tivol EA, Borriello F. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negativeregulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Krumme MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel LF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Yang YF, Zou JP, Mu J. et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57(18):4036–4041. [PubMed] [Google Scholar]
- Kwon ED, Hurwitz AA, Foster BA. et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte–macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95(17):10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ginderachter JA. et al. B7-1, IFN gamma and anti-CTLA-4 co-operate to prevent T-cell tolerization during immunotherapy against a murine T-lymphoma. Int J Cancer. 2000;87(4):539–547. [PubMed] [Google Scholar]
- Hurwitz AA, Foster BA, Allison JP. et al. The TRAMP mouse as a model for prostate cancer. Curr Protocol Immunol. 2001 doi: 10.1002/0471142735.im2005s45. Chapter 20:Unit 20.5. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Foster BA, Kwon ED. et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–2448. [PubMed] [Google Scholar]
- Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor PD. et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22(13-14):1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Cameron F, Whiteside G, Perry C. Ipilimumab: First Global Approval. Drugs. 2011;71(8):1093–1104. doi: 10.2165/11594010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hodi SF. et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. PNAS. 2003;100(8):4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, O’Day S, Urba W, Powderly J, Nichol G, Yellin M. et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- Ribas A, Hauschild A. et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. J Clin Oncol. 2008;26(Supplement):LBA9011. [Google Scholar]
- Ipilimumab. Drugs RD. 2010;10(2):97–110. doi: 10.2165/11584510-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi SF, McDermott DF, Weber RW. et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I. et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- Bakcas T, Mehrishi JN, Moss RW. Ipilimumab (Yervoy) and the TGN142 catastrophe. Immunobiology. [Epub 2011 Jul 7];Immunobiology. doi: 10.1016/j.imbio.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Attarwala H. TGN1412: From Discovery to Disaster. J Young Pharm. 2010;2(3):332–336. doi: 10.4103/0975-1483.66810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmiel KD, Suan D, Liddle C. et al. Resolution of Severe Ipilimumab-Induced Hepatitis after Antithyocyte Globulin Therapy. J Clin Oncol. 2011;29(9):237–240. doi: 10.1200/JCO.2010.32.2206. [DOI] [PubMed] [Google Scholar]
- Minor DR, Chin K, Kashani-Shabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321–325. doi: 10.1089/cbr.2008.0607. [DOI] [PubMed] [Google Scholar]
- Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–830. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia P, Phan GQ, Maker AV. et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE. et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MK, Wolchock JD, Alison J. Anti-CTLA-4 Antibody Therapy: Immune Monitoring During Clinical Development of a Novel Immunotherapy. Semin Oncol. 2010;37(5):473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh L, Kasahara N. et al. The strange case of TGN1412. Cancer Immunol Immunother. 2007;56(2):129–134. doi: 10.1007/s00262-006-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwood JM, Lee S, Moschos SJ. et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/-granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R. et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]