Abstract
Drug-resistant tuberculosis is quickly emerging as one of the largest threats to the global health community. Current chemotherapy for tuberculosis dates back to the 1950s and is arduous, lengthy, and remains extremely difficult to complete in many of the highest burdened areas. This causes inadequate or incomplete treatment, resulting in genetic selection of drug-resistant strains. With a dearth of novel anti-TB drug candidates in the development pipeline, nanoparticle technology allows us to take current chemotherapies and deliver them more efficaciously, reducing the frequency and duration of treatment and increasing bioavailability. This approach can improve patient adherence, reduce pill burden, and shorten time to completion, all which are at the heart of drug resistance. This review examines the multiple advantages of nanoparticle drug delivery of tuberculosis chemotherapy and summarizes the challenges in implementation.
Keywords: tuberculosis, MDR-TB, nanoparticle, ATDs, chemotherapy, drug-resistant tuberculosis
Introduction
Drug resistance in Mycobacterium tuberculosis (Mtb) is widely regarded as one of the most pressing issues the medical community faces today. Mtb infects one-third of the world’s population, of which up to 10 percent will develop active tuberculosis infection (TB) [1]. Treatment for uncomplicated, drug-susceptible TB is strenuous and demanding and has a complex regimen typically requiring a minimum of 6 months of medication [2]. Subsequently, this results in incomplete treatment of chemotherapy for the patient. This incomplete or inadequate treatment has resulted in the emergence of multi-drug-resistant TB (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB). MDR-TB is a strain of TB that is susceptible to few first line medications, and XDR-TB is regarded as untreatable [3]. Both of these conditions are expensive, preventable, and devastating for the person infected. Aims to curb drug resistance, such as implementation of the World Health Organization’s DOTS program (Direct Observed Therapy, short course), have proven problematic and done little to stem the rising cases of DR-TB. While many low- and middle-income countries continue to be plagued with a lack of capacity, the world simply cannot afford to allow the DR-TB epidemics to continue on their current trajectory [4].
Nanoparticle technology, which in the context of drug delivery can be broadly defined as the creation of submicron colloidal particles, has become an exciting advancement in drug delivery [5]. However, to date, little research has been done to elucidate the impact this technology has on the drug-resistant TB epidemic. Although the development of novel TB drugs remains paramount to surmounting the TB epidemic, modifying new drugs in a nanoparticle-based delivery system is a feasible, cost-effective, and readily available alternative. Using current anti-TB drugs, nanoparticle-based formulations may shorten drug regimen duration, reduce frequency, and deliver medications more efficaciously, ultimately reducing patient default and improving completion rates. In turn, this holds significant potential in the reduction of DR-TB cases.
Here, we look at the multiple advantages that nanoparticle delivery of drug-susceptible TB regimens has and its effect on stemming the emergence of drug-resistant TB.
M. tuberculosis, current treatments, and resistance
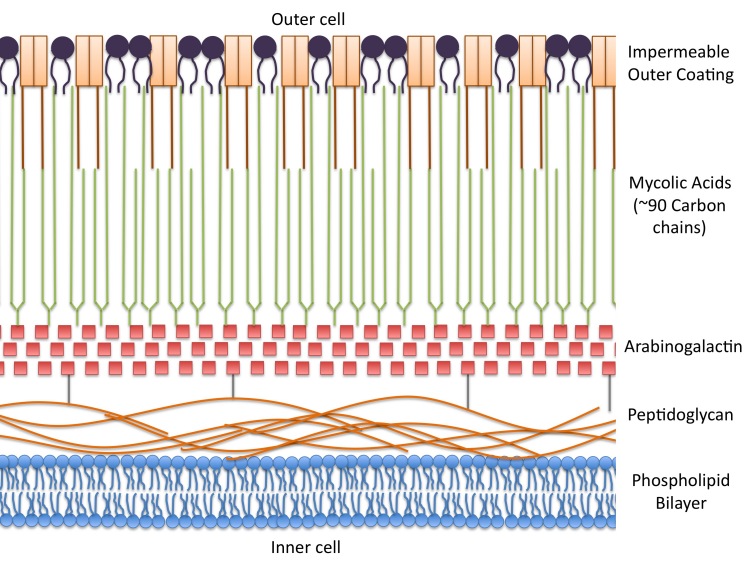
A multifarious treatment for TB is needed due to the particularly tenacious and diverse cell wall of Mtb. This highly complex cell wall is fundamental in the pathogenesis of Mtb, controlling the growth, survival, and the host immunological response. The cell wall is unique among prokaryotes, and its structure has been well-defined in previous literature [6-8]. Briefly, it consists of a layer of peptidoglycan surrounding the cell’s basic lipid bilayer. A second layer of arabinogalactin runs parallel to the peptidoglycan layer, surrounding it in an intricate, sugary shell. A third layer extends perpendicular to the arabinogalactin shell and consists of a complex network of mycolic acids. These long and “sticky” mycolic acids are tightly bound in a final exterior layer, rendering the bacilli virtually impermeable and almost entirely waterproof. This complex armor allows Mtb to be resistant to many antibiotics, avoid death by acidic and alkaline compounds, and prevent cellular phagolysosomal fusion, allowing it to successfully evade lysis. Figure 1 depicts a simple visual representation of the Mtb cell wall.
Figure 1.
Basic structure of M. tuberculosis cell wall. The cell wall of Mtb is comprised of four layers, making treatment difficult and complex. The outer layer of mycolic acids surrounds inner layers of arabinogalactin and peptidoglycan. These surround the traditional phospholipid bilayer of the cell.
These properties make current treatment for active drug-susceptible TB infection particularly arduous; typically a 6-month regimen consisting of a 2-month “intensive” phase of rifampicin (RMP), isoniazid (INH), pyrazinamide (PZA), and ethambutol (EMB) given 7 times per week, followed by a 4-month continuation phase of RMP and INH given 3 times a week [2].
INH, a prodrug activated by the catalaserperoxidase (KatG) enzyme, is the most well-known and used drug to treat TB [9]. Though the activation produces several reactive species that play multiple roles in treating the bacilli, including both reactive oxygen (superoxides, peroxides) and organic (isonicotinic-acyl) species, the primary target of INH is thought to be the enoyl-acyl carrier protein reductase enzyme, which plays a role in the synthesis and elongation of mycolic acids on the cell exterior [10-12]. The bactericidal RMP binds to the β-subunit of the RNA polymerase upstream of the catalytic center and prevents the formation of the RNA chain [9]. PZA is a prodrug converted into pyrazinoic acid (POA) by the pyrazinamidase enzyme [13]. The protonated POA accumulates in the cell and causes cytoplasimic acidification and reduces cell membrane energy, disrupting the proton motive force and affecting membrane transport [14]. EMB is a bacteriostatic agent that inhibits the polymerization of arabinan, arabinogalactan, and lipoarabinomannan, thus preventing its biogenesis formation on the cell wall [15]. Drug resistance in M. tuberculosis is due to spontaneous chromosomal mutations and is worsened by the absence or inefficiency of resistance-mediating genetic elements such as transposons and plasmids [16,17].
Drug resistance in TB is a direct offspring of incomplete or inadequate drug treatment regimens; inadequate treatment of TB regimens often only acts on drug-susceptible strains, allowing drug-resistant species to survive and amplify. Multi drug-resistant TB (MDR-TB) is defined as active tuberculosis disease that is resistant in vitro to the two most highly effective drugs to treat TB ― rifampicin and isoniazid ― with or without resistance to other drugs. Treatment requires the use of several harsh second-line drugs, including injectables such as capreomycin, kanamycin, and amikacin. These drugs have a wide range of severe and chronic side effects, including hearing loss, diarrhea, depression, abdominal pain and nausea, neurapathy, and fatigue [18]. Treatment typically lasts 24 to 27 months and is 25 to 50 times more expensive to treat. Extensively drug-resistant TB (XDR-TB) can be defined as resistance to INH and RMP in vitro, any of the second-line fluoroquinolones, and one or more of the second-line injectable drugs. There is no consensus on treatment regimen, and XDR-TB is generally considered untreatable [3].
Historically, drug resistance in TB is a result of endogenous re-infection, in which incomplete treatment allows for the genetic selection of resistant TB strains. Incomplete treatment may be a result of patient default, inadequate drug supply, or improper diagnosis and is largely perpetuated by poor TB health programs and infrastructures. However, recent evidence has shown that MDR-TB is now being transmitted exogenously, and a growing proportion of MDR-TB cases are now primary acquired infections [19]. This poses a significant threat to health infrastructures, particularly communities living in squalid living conditions with poor infection control.
The scientific community has long been aware of nanoparticle delivery of anti-tuberculosis drugs, yet virtually no literature has highlighted the impact that this technology has on stemming the pressing drug-resistant TB epidemic we face today [20,21]. Nanoparticle-based drug delivery of drug susceptible TB can shorten treatment duration, reduce frequency, and improve treatment outcomes of current therapies, all of which certainly strike at the heart of drug resistance.
Brief overview of nanoparticles in drug delivery
“Nanoparticle” is a broad term that refers to a colloidal particle with a size of less than 1 micron (<1µm) [5]. They can be made from a wide array of biocompatible materials, including natural substances such as alginate and albumin, synthetic substances such as polylactides, or solid lipids. Table 1 shows a list of common polymeric carriers used in nanoparticle drug delivery [22]. Depending on the drug delivery design and matrix composition, nanoparticles will either take the form of monolithic nanoparticles (nanospheres) or nanocapsules. Nanospheres embed the drug in the polymeric matrix, whereas nanocapsules confine the drug within a hydrophobic or hydrophilic core surrounded by a definitive “capsule” [5].
Table 1. Common compounds in nanoparticle drug delivery [22].
| Natural Carriers | Synthetic Carriers |
| Proteins and Polypeptides | Alipatic polyesters and hydroxy acids |
| Albumin | Polyactic Acid |
| Fibrinogen, fibrin | Polyglycolic Acid |
| Collagen | Poly(lactide-co-glycolide) |
| Gelatin | Poly(hydroxybutyric acid) |
| Casein | Polycaprolactone |
|
| |
| Polysaccharides | Polyanhydrides |
| Alginic Acid | Polyorthoesters |
| Starch | Poly(alkylcyanoacrylate) |
| Dextrans, dextrin | Polyamino acids |
| Hydaluronic Acid | Polyacrylamides |
| Chitin | Poly(alkylcarbonates) |
| Chitosan | |
This incredible breadth of diversity among nanoparticle function has proven successful in a wide variety of treatments, including multiple cancer chemotherapies, ARVs, and even suntan lotion [23-25]. In the context of tuberculosis, it provides significant advantage; among the many advantages are increased carrier capacity, reduced degradation in the bowels, improved stability, and the ability to cater to both a hydrophobic and hydrophilic environment. Improved technological versatility also allows for the targeting of nanoparticles to specific cellular processes, affords for the controlled release of medication, and a tailors a specific pharmokenetic profile [22].
Advantages in nanoparticle delivery in drug-susceptible TB drugs
Advantageous Modes of Drug Administration Using Nanoparticle Delivery
Due to the size and versatility of the nanoparticles, drug administration has advantages over standard techniques. Given the wide variety of polymers researched and available for use with tuberculosis chemotherapy, routes of administration include oral, intravenous, subcutaneous, and inhalable. In contrast to current oral drugs, oral delivery of nanoparticle-encapsulated anti-tuberculosis drugs (ATDs) such as poly-(DL-lactide-co-glycolide) (PLG) nanocapsules have been commonly shown to increase efficacy of the administered drugs, reduce degradation in the bowels, and increase uptake and bioavailability [26,27]. The size of the nanoparticle allows for increased transcytosis in the gut lumen’s M cells, facilitated intracellular uptake in the lining epithelium, and improved uptake in the Peyer’s patch [28-30]. This significantly reduces loss of the active anti-TB chemical in the bowels before entering the bloodstream and radically increases bioavailability.
Intravenous administration of first line ATDs is a unique advantage only achieved by nanoparticle technology. Upon administration, this method directly supplies the systemic bloodstream with all ATDs, in effect resulting in absolute bioavailability [22]. Subcutaneous injection of ATD-loaded PLG nanoparticles has also shown similarly high bioavailability in mice [31]. Because of their size, injected or intravenous delivery of nanoparticles have a superior capability of intracapillary passage and cellular uptake, reinforcing exceptional bioavailability [30].
Additionally, nanoparticles also have the potential of inhalable drugs for pulmonary TB, which is the most common form of active tuberculosis disease. In addition to direct delivery of the ATDs to the site of infection, inhaled chemotherapies also do not undergo first-pass metabolism. In addition, nanoparticles are preferentially engulfed by the alveolar macrophage, the same immune cell that first responds to an Mtb encounter [30]. As Mtb is an intracellular organism, releasing ATDs into the “lion’s den” holds significant potential in combating the bacilli.
Sustained Delivery of ATDs Using Nanoparticles
Regardless of delivery method, several studies have shown that nanoparticle delivery of ATDs provides sustained release in both blood plasma as well as organ tissue. Pandley et al. demonstrated in mice that a single orally administered dose of PLG nanoencapsulated ATDs (RMP, INH, and PZA) exhibited superior sustained release with physiologically relevant concentrations maintaining in the blood plasma from 4 days (RMP) to 9 days (INH and PZA), whereas unbound (standard) ATDs were cleared from plasma within 12 to 24 hours. Moreover, physiologically relevant drug concentrations remained in tissue from 9 to 11 days [26]. In a separate experiment, Pandley et al. also demonstrated that when administered via subcutaneous injection, a single dose of drug-loaded PLG nanoparticles resulted in sustained therapeutic blood plasma concentrations for 32 days and tissue concentrations for 36 days [31]. Additionally, Sung et al. demonstrated that an inhalable dry powder of porous nanoparticles containing PA-824 (an alternative anti-TB candidate) sustained drug levels in the lungs for up to 32 hours. On the other hand, lung concentrations of oral administration of PA-824 were considerably less [32].
Targeting of Nanoparticle-Delivered ADTs
Standard delivery of first line ATDs are orally administered once daily, and therefore, anti-TB agent is broadly distributed throughout the body. Moreover, Mtb is an intracellular organism, creating yet another hurdle for these anti-TB agents to overcome. The need for intracellular chemotherapy has been recognized for many years, specifically to the alveolar macrophage, which is a reservoir for Mtb bacilli [30]. Macrophages typically exhibit preferential uptake of nanoparticles [33]. This preferential uptake has been shown in a number of experimental models and is due to cell physiology and the biochemical nature of the nanopolymer [34,35]. This allows for more specific targeting of the active ingredient. Anisimova et al. demonstrate that poly(butyl cyanoacrylate) nanoparticles loaded with INH and streptomycin (another anti-TB candidate) increased the intracellular accumulation of both drugs in human blood monocytes. This is further reinforced by studies showing the improved effect of encapsulated RMP and ciprofloxacin in the infected macrophages [36,37].
Implications of nanoparticle ATD delivery on drug-resistant tuberculosis
Though the advantages outlined above are applied to drug-susceptible TB, they hold significant influence in molding the future of the ever-increasing drug-resistant TB epidemic. Though often implied, this simple fact neglects to be explicitly stated in relevant literature. By increasing bioavailability, obtaining sustained therapeutic plasma and tissue concentrations and targeting discreet intracellular processes, nanoparticles provide a means of reducing treatment duration, frequency, and pill burden.
Unfortunately, lethargy in global drug discovery does not place any novel candidate for first-line treatment for DR-TB on the near horizon. Therefore, the most efficient method of combating DR-TB is proper drug susceptible TB treatment, improved patient compliance, and proper infection control. Chemotherapy regimens with superior sustained release pharmokenetic profiles, targeted delivery, and improved bioavailability can significantly reduce regimen duration, dose frequency, and dose load. This can greatly increase compliance in drug-susceptible TB patients, in turn improving cure rates and eliminating the possibility of a drug resistant reinfection.
However, the true effect of nanoparticle technology can only be appreciated when looking at the broader context of the disease. Though these benefits are universal to TB patients around the globe, they serve particular importance in low- and middle-income countries that have substandard public health infrastructures, squalid living conditions, and poor infection control. These countries carry the majority of the global TB burden, with rates 20 times higher than that of high-income countries [38]. In addition to TB, the burden of DR-TB is also relegated to resource-constrained countries, which remain lacking in proper diagnostic capacity, medical professionals, and access to health care [18]. Additionally, patients with DR-TB often do not have transportation, lack the education, and cannot afford to lose the opportunity cost of missing a day’s work to make the arduous visits to health clinics. It is in these settings that DR-TB thrives and conversely, where nanoparticle delivery of can make the largest impact.
Though there are no first-line MDR- or XDR-TB drugs, nanoparticle technology also can be applied to several second line drugs [22]. However, resistance profiles in DR-TB vary greatly. Recently, there is a growing body of evidence demonstrating that increased levels of drug-susceptible TB chemotherapy, namely high-level INH, can be used to overcome MDR-TB [39,40]. However, this has only been investigated in conventional methods of drug delivery and poses little actual potential; in other words, if the patient defaulted treatment of a standard regimen that resulted in their MDR-TB (regardless of reason), there is little rationale to believe compliance to an increased regimen will prove successful. In this framework, nanoparticle delivery of INH could simultaneously reduce regimen burden while increasing therapeutic dosage of INH. Though increasing drug dose to combat drug resistance is not ideal, in limited-resource settings this development could prove to be life saving ― not just for the patient, but also potentially for the patient’s family and community by reducing the spread of the deadly bacteria.
Potential obstacles and outlook
There are several obstacles to overcome as nanoparticle delivery of TB chemotherapy emerges as a key mediator against both the TB and DR-TB epidemics. Though incomplete treatment is the crux of drug resistance in TB, the cause of this disruption is not always as evident, nor is it readily solvable if it were to be pronounced. Nanotechnology simply has the power to reduce the burden on the patient, a feat that cannot be overstated. However, as a disease of poverty and insufficient health structures, the development of these drugs will not solve the issues of supply, access, or education. Serious and sustained global support to close these health gaps is paramount to the worldwide success of overcoming TB. Elementarily speaking, regardless of efficacy, a drug must be able to reach the patient in order to be effective.
Aside from these factors, nanotechnology remains to have its own barriers to overcome, namely the dearth of human trials for any of the proposed methods. Additionally, physiological barriers persist; for instance, though research is proving successful in some inhalable solutions [41], the mass median aerodynamic diameter often remains too low to allow the particle to reach the needed areas of the lung. Issues of nebulization and insufflation have yet to be ironed out in many proposed models. Moreover, injectable and inhalable routes of administration would require medical professionals and supervision, which is an issue discussed above as already plaguing current infrastructures. Though not all, many polymers would need to stay at a certain temperature, often unfeasible in low-income settings. Quality control measures, shelf life, and long-term stability have yet to be resolved, nor have issues of human toxicity and reaction. These issues would appear to be worked out after the establishment of human trials. Lastly, as a less-profitable business, summoning enough momentum among drug companies and governments will continue to be a hurdle in implementing nanotechnology to TB chemotherapy.
However these obstacles are surmountable, the global health machinery remains lethargic in researching and developing widespread practical uses of nanotechnology in TB and DR-TB. Serious questions remain as to why drug development for TB, in any context, has seen little advance over the past decades; the current chemotherapy regimen used today predates the United States’ first moon landing. Lack of financial and political motivation, a disconnection between the two sciences, and the enduring hope of novel DR-TB drug candidates continue to plague the advancement of nanotechnology in TB chemotherapy.
Once these hurdles are overcome, only deciding on the superior polymer remains in question. Future research will almost certainly focus on elucidating toxicological issues associated with certain polymers, such as more specifically illuminating the fate of the nanocarriers, degradation, and toxicology and routes of elimination of residual polymers. Because of this, natural polymers offer a probable avenue for future research.
Conclusion
There is no magic bullet for surmounting the TB and DR-TB epidemics. However, given our current trajectory in TB programs, research, and drug development, there is little reason to believe that we will turn the tide against the rising DR-TB epidemic in the near future. Though development of novel TB drugs remains a priority, the scarcity of drug candidates in the development pipeline combined with the exponential increase of DR-TB incidence and prevalence forces the medical community to consider plausible alternatives. Nanotechnology is this alternative: it takes current chemotherapy and utilizes it more efficaciously.
The primary advantages are decreased frequency and duration of treatment via sustained concentration profiles and targeted delivery. These advantages will likely improve completion rates by reducing the burden on both the patient and to health infrastructure itself, such as making the DOTS program more manageable and affordable. In contrast to high-income countries where death from DR-TB is virtually non-existent, the importance of this technology in reducing treatment burden in low-income countries cannot be overstated: DR-TB is a death sentence to people in these communities. Moreover, DR-TB is often allowed to run unchecked throughout these regions, perpetuating a deadly and worsening cycle of drug resistance. By using readily available technology, nanoparticle delivery of ATDs provides a logical, cheap, and attractive solution.
Glossary
- TB
tuberculosis
- DR-TB
drug-resistant tuberculosis
- MDR-TB
multi drug-resistant tuberculosis
- XDR-TB
extensively drug-resistant tuberculosis
- Mtb
M. tuberculosis
- DOTS
direct observed therapy, short course
- ATD
anti-tuberculosis drug
- ARV
antiretrovirals
- PLG
poly-(DL-lactide-co-glycolide)
- RMP
rifampicin
- INH
isoniazid
- PZA
pyrazinamide
- EMB
ethambutol
- POA
pyrazinoic acid
References
- Global Tuberculosis Control: Epidemiology, Strategy, Financing. Geneva: World Health Organization; 2009. [Google Scholar]
- Treatment of Drug Susceptible Tuberculosis Disease in Persons Not Infected with HIV. TB Elimination Factsheet: Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention; 2003. [Google Scholar]
- Multidrug and extensively drug resistant TB: 2010 Global Report on surveillance and response. Geneva: World Health Organization; 2010. [Google Scholar]
- Karim SSA, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter J. In: Encyclopedia of Nanoscience and Nanotechnology. Nalwa HS, editor. New York: American Science Publishers; 2004. Nanoparticles as drug delivery system; pp. 161–180. [Google Scholar]
- Jarlier V, Nikaido H. Mycobacterial cell wall: Structure and role in natural resistance to antibiotics. FEMS Microbiology Letters. 1994;123(1-2):11–18. doi: 10.1111/j.1574-6968.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis. 2003;83(1-3):91–97. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- Daffe M, Draper P. In: Advances in Microbial Physiology. Poole RK, editor. Academic Press; 1997. The Envelope Layers of Mycobacteria with Reference to their Pathogenicity; pp. 131–203. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. State of the art series. Drug-resistant tuberculosis. Chiang C-Y, editor. Int J Tuberc Lung Dis. 2009;13(11):1320–1330. [PubMed] [Google Scholar]
- Shoeb HA, Bowman BU, Ottolenghi AC, Merola AJ. Peroxidase-mediated oxidation of isoniazid. Antimicrob Agents Chemother. 1985;27(3):399–403. doi: 10.1128/aac.27.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozwarski DA, Grant GA, Barton DHR, Jacobs WR, Sacchettini JC. Modification of the NADH of the Isoniazid Target (InhA) from Mycobacterium tuberculosis. Science. 1998;279(5347):98–102. doi: 10.1126/science.279.5347.98. [DOI] [PubMed] [Google Scholar]
- Johnsson K. Studies on the mechanism of action of isoniazid and ethionamide in the chemotherapy of tuberculosis. J Am Chem Soc. 1995;117(17):5009. [Google Scholar]
- Scorpio A. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2(6):662. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J Antimicrob Chemother. 2003;52(5):790. doi: 10.1093/jac/dkg446. [DOI] [PubMed] [Google Scholar]
- Mikusova K. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob Agents Chemother. 1995;39(11):2484. doi: 10.1128/aac.39.11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainuddin ZF. Does Mycobacterium tuberculosis have plasmids? Tubercle. 1990;71(1):43. doi: 10.1016/0041-3879(90)90060-l. [DOI] [PubMed] [Google Scholar]
- Telenti A, Imboden P, Marchesi F, Matter L, Schopfer K, Bodmer T. et al. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341(8846):647–651. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- Management of MDR-TB: A Field Guide. Italy: World Health Organization/Partners in Health; 2009. [Google Scholar]
- Andrews JR, Gandhi NR, Moodley P, Shah NS, Bohlken L, Moll AP. et al. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis. 2008;198(11):1582. doi: 10.1086/592991. [DOI] [PubMed] [Google Scholar]
- Zajtchuk R. New technologies in medicine: biotechnology and nanotechnology. Disease-a-Month. 1999;45(11):453–495. doi: 10.1016/s0011-5029(99)90018-4. [DOI] [PubMed] [Google Scholar]
- Florence AT. Issues in Oral Nanoparticle Drug Carrier Uptake and Targeting. J Drug Target. 2004;12(2):65–70. doi: 10.1080/10611860410001693706. [DOI] [PubMed] [Google Scholar]
- Pandey R, Ahmad Z. Nanomedicine and experimental tuberculosis: facts, flaws, and future. Nanomedicine. 2011;7(3):259–272. doi: 10.1016/j.nano.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Perugini P, Simeoni S, Scalia S, Genta I, Modena T, Conti B. et al. Effect of nanoparticle encapsulation on the photostability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. Int J Pharm. 2002;246(1-2):37–45. doi: 10.1016/s0378-5173(02)00356-3. [DOI] [PubMed] [Google Scholar]
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- Govender T, Ojewole E, Naidoo P, Mackraj I. Polymeric Nanoparticles for Enhancing Antiretroviral Drug Therapy. Drug Delivery. 2008;15(8):493–501. doi: 10.1080/10717540802321776. [DOI] [PubMed] [Google Scholar]
- Pandey R, Zahoor A, Sharma S, Khuller GK. Nanoparticle encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. Tuberculosis. 2003;83(6):373–378. doi: 10.1016/j.tube.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Sharma A, Pandey R, Sharma S, Khuller GK. Chemotherapeutic efficacy of poly (dl-lactide-co-glycolide) nanoparticle encapsulated antitubercular drugs at sub-therapeutic dose against experimental tuberculosis. Int J Antimicrob Agents. 2004;24(6):599–604. doi: 10.1016/j.ijantimicag.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Bummer PM. Physical Chemical Considerations of Lipid-Based Oral Drug Delivery-Solid Lipid Nanoparticles. Crit Rev Ther Drug Carrier Syst. 2004;21(1):1–20. [PubMed] [Google Scholar]
- Florence AT, Hussain N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev. 2001;50(Suppl 1):S69–S89. doi: 10.1016/s0169-409x(01)00184-3. [DOI] [PubMed] [Google Scholar]
- Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 2005;172(12):1487–1490. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R. Subcutaneous nanoparticle-based antitubercular chemotherapy in an experimental model. J Antimicrob Chemother. 2004;54(1):266. doi: 10.1093/jac/dkh260. [DOI] [PubMed] [Google Scholar]
- Sung JC. Dry powder nitroimidazopyran antibiotic PA-824 aerosol for inhalation. Antimicrob Agents Chemother. 2009;53(4):1338. doi: 10.1128/AAC.01389-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova YV. Nanoparticles as antituberculosis drugs carriers: effect on activity against Mycobacterium tuberculosis in human monocyte-derived macrophages. Journal of Nanoparticle Research. 2000;2(2):165. [Google Scholar]
- Kayser O, Olbrich C, Croft SL, Kiderlen AF. Formulation and biopharmaceutical issues in the development of drug delivery systems for antiparasitic drugs. Parasitol Res. 2003;90(Suppl 2):S63–S70. doi: 10.1007/s00436-002-0769-2. [DOI] [PubMed] [Google Scholar]
- Pinto-Alphandary H, Andremont A, Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents. 2000;13(3):155–168. doi: 10.1016/s0924-8579(99)00121-1. [DOI] [PubMed] [Google Scholar]
- Barrow ELW. Use of microsphere technology for targeted delivery of rifampin to Mycobacterium tuberculosis-infected macrophages. Antimicrob Agents Chemother. 1998;42(10):2682. doi: 10.1128/aac.42.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz F, Bonini F, Maugein J, Lagueny AM. Ciprofloxacin-loaded polyisobutylcyanoacrylate nanoparticles: pharmacokinetics and in vitro antimicrobial activity. Int J Pharm. 1998;168(2):255–259. [Google Scholar]
- Addressing Poverty in TB control. Geneva: World Health Organization; 2005. [Google Scholar]
- Katiyar SK. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2008;12(2):139. [PubMed] [Google Scholar]
- Leimane V, Riekstina V, Holtz TH, Zarovska E, Skripconoka V, Thorpe LE. et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365(9456):318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- Pandey R. Antitubercular inhaled therapy: opportunities, progress and challenges. J Antimicrob Chemother. 2005;55(4):430. doi: 10.1093/jac/dki027. [DOI] [PubMed] [Google Scholar]