Abstract
Background
Our aim was to summarize our experience with the diagnosis and surgical treatment of solid pseudopapillary neoplasm (SPN) of the pancreas to provide a reference for the management of this rare condition.
Methods
We collected and analyzed retrospective data on the clinical presentation, laboratory investigations, radiologic imaging, pathology and operative details of patients with SPN of the pancreas diagnosed between February 2001 and December 2009.
Results
In all, 23 of 24 patients were women, and the mean age of all patients was 31 years. The most common clinical presentation was vague abdominal pain. Abdominal imaging showed solid or solid cystic masses in the pancreas, mostly in the tail or head of the gland. All patients were treated surgically. There were no postoperative deaths. After follow-up ranging from 4 to 109 months (median 68 mo), 20 of 22 patients who underwent curative resection were alive with no evidence of disease recurrence. Of the 2 patients with R1 resections, 1 died 42 months after surgery, whereas the other underwent a second operation and was alive after 36 months’ follow-up.
Conclusion
Solid pseudopapillary neoplasm of the pancreas is a relatively indolent tumour. The initial diagnosis of SPN of the pancreas is suggested by radiologic imaging findings but should be considered in the context of clinical and histopathologic characteristics. We advocate for complete surgical resection once SPN is diagnosed.
Abstract
Contexte
Nous voulions résumer notre expérience du diagnostic et du traitement chirurgical du néoplasme pseudo-papillaire solide (NPS) du pancréas afin d’établir un point de référence pour la prise en charge de ce problème rare.
Méthodes
Nous avons réuni et analysé des données rétrospectives sur la manifestation clinique, les examens de laboratoire, l’imagerie radiologique, la pathologie et les détails opératoires de patients atteints de NPS du pancréas diagnostiqué entre février 2001 et décembre 2009.
Résultats
Au total, 23 des 24 patients étaient des femmes et l’âge moyen de tous les patients s’établissait à 31 ans. La manifestation clinique la plus courante était une vague douleur abdominale. L’imagerie abdominale a révélé la présence de masses solides ou kystiques solides au pancréas, surtout à la queue ou à la tête de la glande. Tous les patients ont été traités par chirurgie. Il n’y a pas eu de décès après l’intervention. Après un suivi échelonné sur 4 à 109 mois (médiane de 68 mois), 20 des 22 patients qui ont subi une résection curative étaient vivants et ne présentaient aucun signe de réapparition de la maladie. Des 2 patients qui ont subi une résection R1, 1 est décédé 42 mois après l’intervention tandis que l’autre a subi une deuxième intervention et était vivant après un suivi de 36 mois.
Conclusion
Le néoplasme pseudo-papillaire solide du pancréas est une tumeur relativement indolente. Le diagnostic initial de NPS du pancréas est indiqué par les résultats d’imagerie radiologique, mais il faudrait l’envisager en présence de caractéris-tiques cliniques et histopathologiques. Nous préconisons la résection chirurgicale complète après le diagnostic.
A solid pseudopapillary neoplasm (SPN) of the pancreas is a rare pancreatic tumour. It was first described by Dr. Frantz in 1959,1 and was defined by the World Health Organization (WHO) in 1996 as “solid pseudopapillary tumours” of the pancreas. As a rule, these neoplasms have a relatively low malignant potential and are diagnosed mostly in young women. The usual treatment is surgical resection; after resection, the prognosis is generally favourable. However, it can be difficult to diagnose these tumours as they tend to manifest with only vague and nonspecific abdominal symptoms.
In this study, we report our recent experience with SPN of the pancreas and include a summary of the current literature to provide a reference for the management of this rare disease.
Methods
We retrospectively reviewed the cases of consecutive patients who underwent surgery for pathologically confirmed SPN of the pancreas at the Departments of General Surgery, Affiliated Second Hospitals of Sun Yat-sen University and Tongji University between February 2001 and December 2009. We evaluated patients’ demographic characteristics, clinical presentation, radiologic imaging findings, surgical treatment, perioperative complications, pathology and long-term survival. Pathologically, the diagnosis of SPN of the pancreas was based on the gross and microscopic appearance as well as immunohistochemical staining. We obtained information on follow-up from out-patient notes. When patients did not have a recent (within 3 mo) outpatient visit, we contacted them or their families by telephone for further follow-up. The ethical approval was obtained from our hospital review board, and all patients were informed and agreed to participate in our research.
Results
Participants
In total, 24 patients underwent surgery for SPN of the pancreas during the study period. Of these patients, 23 (95.8%) were female and 1 (4.2%) was male. Patient age ranged from 11 to 61 years. Fourteen female patients were younger than 30 years, and the mean age of all patients was 31 years. The patients had a median symptom duration of 4 weeks (range 3 d to 15 mo). Symptomatology was generally nonspecific: vague abdominal pain was the most common symptom (10 patients, 41.7%), with a palpable abdominal mass being the second most common symptom (8 patients, 33.3%). Serum carcinoembryonic antigen was elevated in 1 (4.2%) patient, serum cancer antigen (CA) 19–9 was elevated in 2 (8.3%) patients, and serum CA 72–4 was elevated in 1 (4.2%) patient. Patient characteristics are summarized in Table 1.
Table 1.
Characteristics of patients with solid pseudopapillary neoplasms of the pancreas
| Characteristic* | No. (%) |
|---|---|
| Pain | 10 (41.7) |
| Mass | 8 (33.3) |
| Asymptomatic | 5 (20.8) |
| Discomfort | 3 (12.5) |
| Vomiting | 2 (8.3) |
| Nausea | 1 (4.2) |
| Weight loss | 1 (4.2) |
| Jaundice | 1 (4.2) |
| Diarrhea | 1 (4.2) |
| Anorexia | 1 (4.2) |
| Age, yr | |
| < 30 | 14 (58.3) |
| ≥ 30 | 10 (41.7) |
| Sex | |
| Female | 23 (95.8) |
| Male | 1 (4.2) |
| Tumour diameter, cm | |
| < 5 | 3 (12.5) |
| 5–10 | 16 (66.7) |
| ≥ 10 | 5 (20.8) |
| Tumour location | |
| Tail | 8 (33.3) |
| Head | 7 (29.2) |
| Head and body | 3 (12.5) |
| Body and tail | 3 (12.5) |
| Body | 2 (8.3) |
| Processus anconaeus | 1 (4.2) |
| Operation | |
| R0 resections | 22 (91.7) |
| R1 resections | 2 (8.3) |
| Regular follow-up | 22 (91.7) |
Some patients had compound symptoms.
Imaging results
Patients underwent a variety of radiologic investigations, including transabdominal ultrasonography (n = 24), computed tomography (CT, n = 24) and magnetic resonance imaging (MRI, n = 6; Fig. 1). Ultrasounds showed a hypoechoic, clear-bordered cystic or cystic–solid mass in all 24 patients and capsular integrity in 16 (66.7%) patients. Colour Doppler flow imaging showed a vascular signal in the mass in 5 (20.8%) patients, with enhanced blood flow signal around the tumour in 3 (12.5%) patients. Computed tomography scans showed a clear-bordered cystic or cystic–solid mass with heterogeneous density in all 24 patients and satellite calcification around the mass in 4 (16.7%) patients. There was enhancement of the solid part of the tumour and the capsule in 23 (95.8%) patients. In all 6 patients who underwent MRI, there was heterogeneous hyperintensity of the tumour on T1-weighted images with a higher T2 signal and patchy enhancement of the internal part of the tumour. Solid pseudopapillary neoplasm was considered in the differential diagnosis for all patients, but the diagnosis was not definitive in any of them. In most patients, an indeterminate cystic neoplasm of the pancreas was diagnosed. The single male patient originally received a misdiagnosis of a chromaffin-cell tumour.
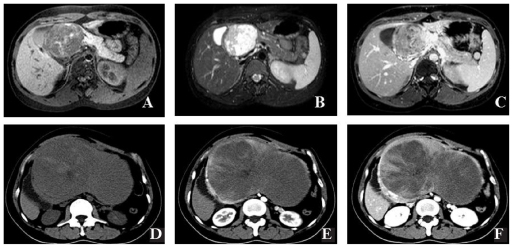
Fig. 1.
Imaging diagnosis of solid pseudopapillary neoplasm of the pancreas. (A) Magnetic resonance imaging (MRI), T1-weighted imaging shows that the tumour is a well-marginated, large, encapsulated, solid and cystic mass with areas of hemorrhagic degeneration, as revealed by high signal intensity. (B) T2-weighted image: papillary high signal and irregular low signal intensities were observed. (C) The most common enhancement patterns were high signal intensity on T1-weighted imaging and slightly progressive heterogeneous peripheral contrast enhancement after administration. (D) Plain computed tomography (CT) scanning shows an encapsulated lesion with well-defined borders and variable central areas with cystic degeneration, necrosis or hemorrhage. (E) Contrast-enhanced CT scan shows a well-encapsulated but lobulated mass within the body and tail of the pancreas. The mass has mixed attenuation, with predominantly solid soft-tissue attenuation noted within the cephalic portion of the mass, which is associated with scattered punctate calcifications. Slight enhancement of the cystic wall and solid part was observed in the arterial phase. (F) The portal venous phase demonstrates obvious papillae-like enhancement of the solid portion and central cleft-like unenhanced cystic region of the mass.
Surgical treatment and complications
All 24 patients underwent surgical exploration. The most common location of the tumour was the tail of the pancreas (8 patients, 33.3%) followed by the head (7 patients, 29.2%), both the head and body (3 patients, 12.5%), both the body and tail (3 patients, 12.5%), the body alone (2 patients, 8.3%) and the processus anconaeus (1 patient, 4.2%). The mean diameter of the tumour was 7.5 (range 2.3–25.9) cm. Two (8.3%) patients had metastases at the time of exploration: 1 had invasion into the portal vein and 1 had metastasis at the omentum and abdominal cavity. Of the patients with lesions in the head and/or in the neck, 6 underwent pancreaticoduodenectomy. One of these patients underwent a simultaneous partial liver resection (resection of metastases). Nine of the patients with lesions in the tail (or the body) underwent distal pancreatectomy, 2 of them with splenectomy. Of the remaining 9 patients, a local resection was performed in 6 and enucleation in 3.
Twenty-two patients had R0 resections, whereas 2 had R1 resections. Of the 2 patients with R1 resections, 1 patient had a tumour in the head of the pancreas and underwent a local resection. This patient was found to have focal invasion of the resection margin by tumour cells. The other patient who underwent R1 resection had a tumour in the tail and underwent distal pancreatectomy, splenectomy and partial omentectomy and was found to have multiple metastases in the abdominal cavity. The metastases were noted at the time of surgery and confirmed by pathologic review. This patient died 42 months after exploration.
There were no perioperative surgical deaths. Perioperative complications occurred in 10 (41.7%) patients. Three patients had intra-abdominal abscesses; 2 were treated with CT-guided percutaneous drainage and the third underwent noninvasive treatment. Three patients had a pancreatic fistula, as defined by a drain output of high amylase fluid (greater than 3 times the upper limit of serum amylase) for more than 3 days postoperatively. Three patients had superficial wound infections and 1 patient who underwent splenectomy had thrombocythemia. The median postoperative stay in hospital was 9 (range 6–42) days.
Pathology
The gross appearance of the resected neoplasms had a mixed solid and cystic appearance with varying proportions of each component (Fig. 2). The cut surface showed large spongy areas of hemorrhage alternating with both solid and cystic degeneration. We noted degenerative changes in all 24 lesions. Two of 24 patients had metastases in the portal vein, omentum and abdominal cavity. A single patient had evidence of lymph node involvement. Immunohistochemical evaluation was performed on 21 specimens (Table 2, Fig. 3). The immunohistochemical analysis demonstrated vimentin, alpha-1-antitrypsin (AAT) and neuron specific enolase (NSE), spotted expressed chromogranin (CgA) and synaptophysin (Syn).
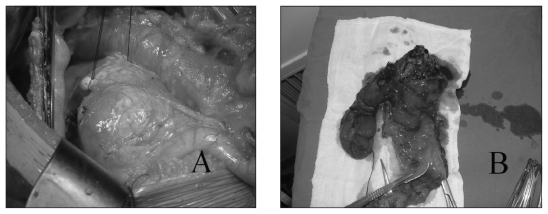
Fig 2.
The solid pseudopapillary neoplasm (SPN) in a 20-year-old woman was located at the head of the pancreas; its size was 7.1 × 5.3 cm. (A) We performed a Whipple operation for a complete excision. (B) After the operation, we cut the neoplasm open and found the gross appearance of the resected neoplasms had a mixed solid and cystic appearance with varying proportions of each component. The patient had no specific clinical symptoms other than an abdominal mass.
Table 2.
Immunohistochemical staining of solid pseudopapillary neoplasm of the pancreas
| Stain | Strong+* | Focal+ | Negative |
|---|---|---|---|
| Neuron specific enolase | 18 | 2 | 1 |
| Chromogranin A | 8 | 3 | 10 |
| Synaptophysin | 3 | 10 | 8 |
| Vimentin | 21 | 0 | 0 |
| Cytokeratin | 0 | 3 | 18 |
| Creatine kinase | 1 | 5 | 15 |
| Alpha-1-antitrypsin | 19 | 2 | 0 |
In some samples, a few cells showed S-100(+).
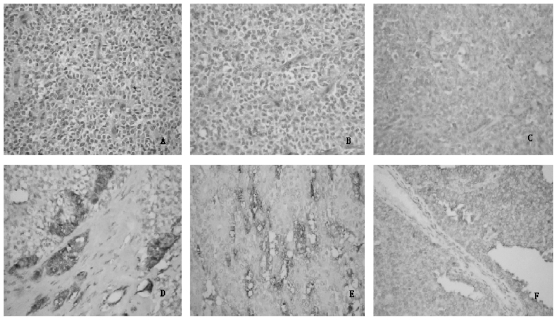
Fig 3.
Immunohistochemical staining of solid pseudopapillary neoplasm (SPN) of the pancreas (all hema-toxylin and eosin staining, original magnification ×200). (A) Histologic view of SPN shows pseudopapillary structure with a fibrovascular core and mucous degeneration. The neoplastic epithelial cells were polygonal in form, consistent in size and noncohesive. (B) Metastases or invasions of solid masses had a higher nuclear grade and more prominent necrobiotic nests characterized by aggregates of cells with pycnotic nuclei and eosinophilic cytoplasm. Immunohistochemical evaluation of the SPN showing (C) neuron specific enolase(+), (D) synaptophysin(+), (E) alpha-1-antitrypsin(+) and (F) vitmentin(+).
Long-term outcome
Follow-up schedule was dictated by each patient’s surgeon and usually involved biannual to annual clinical exams with either CT or ultrasonography. Two (8.3%) patients were completely lost to follow-up after curative pancreatectomy. The remaining 22 patients had regular follow-up with a mean duration of 68 (range 4–109) months. At the time of writing, 20 of the 22 patients who underwent curative resection were alive with no evidence of disease recurrence. Of the 2 patients with R1 resections for malignant SPN of the pancreas, 1 patient with multiple metastases in the abdominal cavity died 42 months after surgical exploration and resection. The other patient with microscopically positive margins was found to have metastases in the liver 32 months after surgery. This patient underwent further exploration and resection of all evident metastases followed by treatment with adjuvant cisplatin and 5-fluorouracil for 6 months. The patient has remained disease-free for 4 years since the second operation.
Discussion
Solid pseudopapillary neoplasm of the pancreas is considered to be a low-grade malignant tumour. The tumour has been identified by a number of synonyms, including Frantz’s tumour, solid and cystic acinar tumour, papillary epithelial neoplasm, and solid and papillary epithelial tumour or neoplasm. Our data show that most tumours are located in the pancreatic tail and head, which is supported by other studies.2 The cellular origin of the tumours is unclear and might involve ductal cells, acinar cells, endocrine cells or multipotential stem cells. Tumour recurrence is a distinct possibility after surgery and mandates routine follow-up with imaging.3 The most common sites of metastasis are the liver, regional lymph nodes, mesentery, omentum and peritoneum.4 In our series, 2 of 24 (8.3%) patients had tumour recurrence. Metastases were rarely seen and occurred mainly in the liver and omentum. The tumour also can invade the vessels near it, such as the superior mesenteric vein or portal vein. In our study, 2 (8.3%) patients had liver and omentum invasion. We found that clinical factors, such as sex; age; symptoms; and tumour size, location and characteristics were not closely related to its malignant potential. Our findings are generally similar to those found in the literature (Table 3), though it should be noted that SPN is a rare tumour and that the presentation of individual cases can be unique.
Table 3.
Comparison between the clinical features and outcome of solid pseudopapillary neoplasms in the present study with those reported in 4 large previous studies
| Study | No. | Age, mean (range) yr | Sex ratio | Tumour size, mean (range) cm | Follow-up, mean (range) mo | No. metastases | Survival, % |
|---|---|---|---|---|---|---|---|
| Salvia et al.2 | 31 | 34 (7–56) | 27 F : 4 M | 5.4 (2–20) | 58 (12–229) | 0 | 100 |
| Tipton et al.5 | 14 | 30 (15–57) | 13 F : 1 M | 7.0 (4–16) | 88 (3–240) | 2 | 100 |
| Goh et al.6 | 16 | 30 (14–53) | 15 F : 1 M | 9.5 (5–24) | 67 (3–186) | 0 | 100 |
| Yang et al.7 | 26 | 32 (15–64) | 22 F : 4 M | 6.25 (2–15) | 33 (3–69) | 2 | 96.2 |
| Present study | 24 | 31 (11–61) | 23 F : 1 M | 7.5 (2–26) | 68 (4–109) | 2 | 95.8 |
F = female; M = male.
Solid pseudopapillary neoplasm of the pancreas is a rare disease with a reported incidence of 0.1%–5.0% of all pancreatic tumours. The patients are often young women presenting with an abdominal mass. In our series, most patients presented with vague and nonspecific abdominal symptoms, including vague pain in the stomach and a palpable mass. We observed that patients often described their symptoms as an “indescribable” abdominal distension. Owing to these subtle symptoms, diagnosis is frequently delayed and tumour size at presentation is frequently large. Symptoms due to compression are possible, and gastric outlet obstruction is a possibility.8 Generally speaking, there is no specific clinical syndrome for SPN of the pancreas, and the disease tends to be misdiagnosed, especially in male patients. In our study, the male patient received a misdiagnosis of chromaffin-cell tumour. Usually there is no evidence of pancreatic insufficiency, abnormal liver function tests, cholestasis, elevated pancreatic enzymes or an endocrine syndrome. Tumour markers are also generally unremarkable.9 A diagnosis of SPN of the pancreas should be considered, especially if the patient is a young woman with no other cause for pancreatic masses, and the diagnosis should not be discounted in male patients.
Solid pseudopapillary neoplasms of the pancreas are readily diagnosable based on characteristic histologic and cytologic appearance. They can demonstrate a variety of histologic structures, including solid, pseudopapillary and cystic regions. The neoplastic cells are uniform and polygonal with abundant cytoplasm and contain oval, regular nuclei. Cellular heteromorphism and mitotic phase can be demonstrated in some patients. Distinction from acinar carcinoma and islet cell neoplasm should be made in conjunction with immunohistochemistry. Negative CgA and Syn staining essentially rules out an islet cell neoplasm, whereas a negative keratin stain excludes acinar carcinoma. Immunohistochemical analysis in the present series showed strong staining for NSE (18 patients, 75.0%), vimentin (21 patients, 87.5%) and AAT (19 patients, 79.2%). These results suggest that NSE, vimentin and AAT may be effective indicators of SPN. However, expression of most immunohistochemical markers has nothing to do with the malignant potential of SPN. A recent pilot study reported that SPN of the pancreas always showed cytoplasmic/nuclear accumulation of β-catenin and frequent expression of cyclin-D1 and that tumour size was closely related to microscopic features of malignant potential and apparently has an inverse relation with the expression of cyclin-D1 and Ki-67.10 Yang and colleagues7 reported that in 2 of 26 patients with malignant SPN, the expression of Ki-67 was positive; however, expression of K1-67 was negative in the 24 patients with nonmalignant SPN, suggesting that Ki-67 might be a marker of malignant potential. In our further research, we will explore the relation between Ki-67 and malignant potential of SPN tumours.
Radiologic imaging is an essential part of the diagnosis of pancreatic SPN. Abdominal ultrasonography with Doppler examination can be very useful for locating the mass to the pancreas and detecting low blood flow around the tumour. Computed tomography is also a very useful technique for diagnosing an SPN, and enhanced CT must be performed. In general, SPN tumours demonstrate no enhancement of the cystic portions but slight enhancement of the solid portions in the arterial phase and marked enhancement in the portal venous phase.11 When compared with MRI, CT has inherent limitations in showing certain tissue characteristics, such as hemorrhage, cystic degeneration or the integrity of the tumour capsule, that would be suggestive of SPN.12 Because of its superior contrast resolution, MRI scans display a capsule and intratumoural hemorrhage better than CT scans. We feel that MRI is the most effective way to diagnose SPN of the pancreas. This tumour should be considered if a mass is found to be well-marginated, large, encapsulated, solid and cystic, with areas of hemorrhagic degeneration, as revealed by high signal intensity on T1-weighted imaging and progressive peripheral or slightly heterogeneous contrast enhancement.13 The results of our study show that radiologic imaging, particularly when combined with clinical features, such as age and sex, raises the possibility of an SPN tumour and is enough to indicate surgery without the need for a preoperative biopsy. Another point of emphasis in the radiologic assessment of these tumours is their size. Large tumours (> 3.0 cm) more commonly display many of the imaging characteristics of SPNs, but smaller tumours (< 3.0 cm) often are atypical for SPNs. We advocate for the use of MRI or percutaneous biopsy in diagnosing small SPNs.14
Surgery is the best curative treatment for SPN of the pancreas.2,15 A complete, R0 resection should be the goal of surgery. Small tumours with complete capsules can be considered for local resection or enucleation. Tumour size has not been shown to be a predictor of respectability, as large tumours are frequently still operable and surgery is the treatment of choice even in the cases of distant hepatic metastasis or local recurrence, which are not contraindications for surgical therapy.16,17 In our study, the 2 patients with tumour metastases underwent tumour resection; 1 of these patients was alive with no evidence of tumour recurrence more than 4 years after surgery. There are other treatment modalities for liver metastasis, such as alcohol injection, transarterial chemoembolization, γ-radiation therapy and even liver transplantation.18 For the unresectable SPNs (too large or widespread metastasis), radio-therapy is suggested, as the tumours are radiosensitive.19 There has not been consistent agreement about the diagnostic criteria for malignant SPN. Considering our data and the results of previous studies (Table 3), we suggest that the following characteristics indicate malignant SPNs:
gross capsular invasion;
histologic findings of high nuclear grade, cellular pleomorphism, venous invasion and necrobiotics; and
immunohistochemistry findings of expression of Ki-67.
In the present study, curative resection was possible in the 22 patients with minimal morbidity and no deaths, so exploration for potential resection seems appropriate in almost all patients regardless of the size of the tumour mass.
Generally speaking, the prognosis of patients with SPN is good, even with local recurrence and metastasis. Most cases of SPN are limited to the pancreas and should be resected. Recurrence, local invasion and limited metastasis are not necessarily contraindications for resection, and some patients with unresectable SPNs may also have a long survival time.20 The overall 5-year survival rate of patients with SPN is about 95%.19
Conclusion
Solid pseudopapillary neoplasm of the pancreas is a kind of low-grade malignant tumour and is prone to occur in young women. The diagnosis should be considered in a young woman with a pancreatic mass and vague abdominal symptoms. The correct diagnosis of SPN of the pancreas depends on comprehensive analysis of clinical features, imaging and histopathologic characteristics. Surgical resection should be performed and should be considered even in patients with advanced disease.
Acknowledgments
We are grateful to all colleagues at the Department of General Surgery, the Affiliated Second Hospital of Sun Yat-sen University and Tongji university for providing surgical care for these patients.
Footnotes
Competing interests: None declared.
Contributors: Drs. Guo, R.F. Chen and Zou designed the study. Drs. Zhou and J.S. Chen acquired the data, which Drs. Zhou, Li, Lin and Wang analyzed. Drs. Guo, Zhou and Lin wrote the article, which Drs. R.F. Chen, Zou, Li, Wang and J.S. Chen reviewed. All authors approved its publication.
References
- 1.Zinner MJ, Shurbaji MS, Cameron JL. Solid and papillary epithelial neoplasms of the pancreas. Surgery. 1990;108:475–80. [PubMed] [Google Scholar]
- 2.Salvia R, Bassi C, Festa L, et al. Clinical and biological behavior of pancreatic solid pseudopapillary tumors: report on 31 consecutive patients. J Surg Oncol. 2007;95:304–10. doi: 10.1002/jso.20685. [DOI] [PubMed] [Google Scholar]
- 3.Sclafani LM, Reuter VE, Coit DG, et al. The malignant nature of papillary and cystic neoplasm of the pancreas. Cancer. 1991;68:153–8. doi: 10.1002/1097-0142(19910701)68:1<153::aid-cncr2820680128>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Huang HL, Shih SC, Chang WH, et al. Solid-pseudopapillary tumor of the pancreas: clinical experience and literature review. World J Gastroenterol. 2005;11:1403–9. doi: 10.3748/wjg.v11.i9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tipton SG, Smyrk TC, Sarr MG, et al. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 2006;93:733–7. doi: 10.1002/bjs.5334. [DOI] [PubMed] [Google Scholar]
- 6.Goh BK, Tan YM, Cheow PC, et al. Solid pseudopapillary neoplasms of the pancreas: an updated experience. J Surg Oncol. 2007;95:640–4. doi: 10.1002/jso.20735. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Jin C, Long J, et al. Solid pseudopapillary tumor of the pancreas: a case series of 26 consecutive patients. Am J Surg. 2009;198:210–5. doi: 10.1016/j.amjsurg.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 8.Siech M, Merkle E, Mattfeldt T, et al. [Solid pseudopapillary tumors of the pancreas] [Article in German] Chirurg. 1996;67:1012–5. doi: 10.1007/pl00002511. [DOI] [PubMed] [Google Scholar]
- 9.Yu PF, Hu ZH, Wang XB, et al. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010;16:1209–14. doi: 10.3748/wjg.v16.i10.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brázdil J, Hermanova M, Kren L, et al. [Solid pseudopapillary tumor of the pancreas: 5 case reports] [Article in Czech] Rozhl Chir. 2004;83:73–8. [PubMed] [Google Scholar]
- 11.Dong DJ, Zhang SZ. Solid-pseudopapillary tumor of the pancreas: CT and MRI features of 3 cases. Hepatobiliary Pancreat Dis Int. 2006;5:300–4. [PubMed] [Google Scholar]
- 12.Kehagias D, Smyrniotis V, Gouliamos A, et al. Cystic pancreatic neoplasms: computed tomography and magnetic resonance imaging findings. Int J Pancreatol. 2000;28:223–30. doi: 10.1385/IJGC:28:3:223. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann KA, Helmberger T, Bruns C, et al. [Solid pseudopapillary pancreas tumors — often neglected] [Article in German] Radiologe. 2008;48:764–9. doi: 10.1007/s00117-008-1673-2. [DOI] [PubMed] [Google Scholar]
- 14.Yao X, Ji Y, Zeng M, et al. Solid pseudopapillary tumor of the pancreas: cross-sectional imaging and pathologic correlation. Pancreas. 2010;39:486–91. doi: 10.1097/MPA.0b013e3181bd6839. [DOI] [PubMed] [Google Scholar]
- 15.Alexandrescu DT, O’Boyle K, Feliz A, et al. Metastatic solid-pseudopapillary tumour of the pancreas: clinico-biological correlates and management. Clin Oncol (R Coll Radiol) 2005;17:358–63. doi: 10.1016/j.clon.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Gedaly R, Toledano A, Millan G, et al. Treatment of liver metastases from a solid pseudopapillary tumor of the pancreas. J Hepatobiliary Pancreat Surg. 2006;13:587–90. doi: 10.1007/s00534-006-1122-4. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer CM, Dixon E, Grant D. Management of a solid pseudopapillary tumor of the pancreas with liver metastases. HPB (Oxford) 2003;5:264–7. doi: 10.1080/13651820310001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumida W, Kaneko K, Tainaka T, et al. Liver transplantation for multiple liver metastases from solid pseudopapillary tumor of the pancreas. J Pediatr Surg. 2007;42:e27–31. doi: 10.1016/j.jpedsurg.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 19.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965–72. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Mao C, Guvendi M, Domenico DR, et al. Papillary cystic and solid tumors of the pancreas: A pancreatic embryonic tumor? Studies of three cases and cumulative review of the world’s literature. Surgery. 1995;118:821–8. doi: 10.1016/s0039-6060(05)80271-5. [DOI] [PubMed] [Google Scholar]