Abstract
Vaccines play a vital role in protecting the host against infectious disease. The most effective licensed vaccines elicit long-term antigen-specific antibody responses by plasma cells in addition to the development of persisting T cell and B cell memory. The relative contributions of these different immune cell subsets is context-dependent and varies depending on the attributes of the vaccine (i.e., live/attenuated, inactivated, subunit) as well as the biology of the pathogen in question. For relatively simple vaccines against bacterial antigens (e.g., tetanus toxin) or invariant viruses, the immunological correlates of protection are well-characterized. For more complex vaccines against viruses, especially ones that mutate or cause latent infections, it is more difficult to define the specific correlates of immunity. This often requires observational/natural history studies, clinical trials, or experimental evaluation in relevant animal models in order for immunological correlates to be determined or extrapolated. In this review, we will discuss the relative contributions of virus-specific T cell and B cell responses to vaccine-mediated protection against disease.
Introduction
Vaccines play a fundamental role in modern medicine and the introduction of Edward Jenner’s smallpox vaccine in 1798 marked an important turning point in the battle against infectious disease (Jenner, 1798). With the notable exceptions of smallpox and rabies, many of the early advances made in vaccinology during the 18th and 19th century were focused primarily on bacterial pathogens (Plotkin and Plotkin, 2008). These initial studies reflect the tools that were developed by early microbiologists to grow and study important pathogenic bacteria as well as some of the challenges faced by virologists prior to the advent of modern tissue culture technologies. During the 20th century, new viral vaccines against yellow fever, influenza, polio, measles, mumps, rubella, and others emerged. Today, there are now 14 vaccines licensed in the US that are directed against viral pathogens (Table 1) (FDA, 2010).
Table 1.
Correlates and surrogates of vaccine-mediated immunity to virusesa
| Vaccine | Vaccine type | Test | Protective level | Reference |
|---|---|---|---|---|
| Hepatitis A | Inactivated | ELISA | 10 mIU/mL | (Fiore, Finestone, and Bell, 2008; Nalin et al., 1993) |
| Hepatitis B (HbsAg) | Protein | ELISA | 10 mIU/mL | (Jack et al., 1999) |
| Human papillomavirus | Virus-like particles | ELISA | NDb | (Schiller, Frazer, and Lowy, 2008) |
| Influenza | Inactivated/live attenuated | HAI | 1:40 titer | (Dowdle et al., 1973) |
| Japanese encephalitis virus | Inactivated/live attenuated | Neutralization | 1:10 titer | (Hombach et al., 2005) |
| Measles | Live attenuated | Neutralization | 120–200 mIU/mL | (Chen et al., 1990; Samb et al., 1995) |
| Mumps | Live attenuated | Neutralization? | ND | (Weibel et al., 1975) |
| Polio | Live attenuated/Inactivated | Neutralization | 1:4 to 1:8 titer | (Plotkin and Vidor, 2008) |
| Rabies | Inactivated | Neutralization | 0.5 IU/mL | (WHO, 2007) |
| Rotavirus | Live attenuated | Serum IgA | ND | (Franco, Angel, and Greenberg, 2006) |
| Rubella | Live attenuated | Immunoprecipitation | 10–15 mIU/mL | (Matter, Kogelschatz, and Germann, 1997; Skendzel, 1996) |
| Smallpox | Live attenuated | Neutralization | 1:20 to 1:32 titer | (Mack, Noble, and Thomas, 1972; Sarkar, Mitra, and Mukherjee, 1975) |
| Varicella Varicella Zoster | Live attenuated | FAMA, gpELISA T cell proliferation | 1:64 titer, 5 IU/mL ND | (Li et al., 2002; White et al., 1992) (Weinberg et al., 2009) |
| Tick-borne encephalitis virus | Inactivated | Neutralization | 125 IU/mL | (Kreil et al., 1997) |
| Yellow Fever | Live attenuated | Neutralization | 0.7 LNI | (Mason et al., 1973) |
Adapted from (Plotkin, 2008; Plotkin, 2010). The correlates and surrogates of immunity for most vaccines are based on serum antibody titers, but several vaccines also induce mucosal immunity and/or T cell-mediated immune responses.
ND, not defined.
Development of an effective vaccine is only the first step in controlling or eradicating a particular disease. For instance, despite the development of an effective measles vaccine in the 1960s, this virus is still a significant health threat worldwide, with an estimated 10% of deaths in children less than 5 years of age attributed to complications from measles (CDC, 1998). In the pre-vaccine era, measles was endemic in the U.S. with ~500,000 reported cases each year (Amanna and Slifka, 2005). Following the introduction of measles vaccination, the number of cases has dropped dramatically (Amanna and Slifka, 2005) – with only 127 cases reported in 2008 (Hall-Baker et al., 2010). Importantly, of those reporting measles, 78% (99/127) were unvaccinated, often for personal or religious beliefs. Most of these cases of measles were concentrated among groups of unvaccinated individuals who rapidly spread the disease through their local community (Hall-Baker et al., 2010). Fortunately, despite these small outbreaks, measles is still well controlled at a population level. In the past, introduction of a viral pathogen could decimate a naive population, often with a disproportionate effect on vulnerable populations including the very young and very old (Siegrist, 2008). This does not occur in the context of high vaccination coverage (e.g., measles vaccination in the U.S.) due in large part to herd immunity. When vaccination coverage is high, the transmission of contagious disease is reduced and this protects vulnerable individuals from encountering the pathogen and becoming infected. The goal of herd immunity is to attain immunization levels that stop the endemic spread of transmissible diseases, which may require immunization rates of >90% for some of the most contagious viruses, such as measles (Fine and Mulholland, 2008). Herd immunity is critical from a public health perspective because often the most vulnerable populations in a community (e.g., infants, elderly, immunocompromised) are also the least likely to respond effectively to vaccination. This is an increasingly important topic, considering that the proportion of elderly individuals in the world is expected to rise from 10% in 2000 to 21% in 2050 (Aspinall et al., 2007).
Continued innovation in vaccine research has resulted in a relatively large number of antiviral vaccines that have been licensed, as well as a number more that are in various stages of development (Table 1). Production of a successful vaccine is not a trivial process and requires a strong foundation based on safety, feasibility, cost, and above all, proof of protective efficacy (Figure 1). These basic tenets have factored into the research and development of essentially all licensed vaccines, including the very first vaccine developed by Edward Jenner (Jenner, 1798). After describing substantial epidemiological evidence showing that natural cowpox infection [or perhaps, horsepox; (Dasgupta et al., 2007; Tizard, 1999; Tulman et al., 2006)] could protect against smallpox, Jenner began his studies by performing Variolation (inoculation of smallpox, i.e., Variola virus, into the skin). This approach was used successfully to determine if prior cowpox infection could indeed protect against Variola. As early as 1795, Jenner noted, “…though the variolous matter was repeatedly inserted into his arm, I found it impracticable to infect him with it; an efflorescence only, taking on an erysipelatous look about the centre, appearing on the skin near the punctured parts. During the whole time that his family had the smallpox, one of whom had it very full, he remained in the house with them, but received no injury from exposure to the contagion” (Jenner, 1798). This provided important proof-of-principle, but to demonstrate actual vaccine-mediated protection, Jenner experimentally infected subjects with cowpox by scarification and then performed variolation. Similar to natural cowpox infection, direct vaccination with cowpox provided protection against Variola virus. This not only demonstrated protective efficacy, but because cowpox was far more attenuated and safer to use than variolation (the standard of care at that time), Jenner had greatly increased the safety of his approach. In terms of technical feasibility (as well as safety), Jenner was very careful to train other physicians in the art of scarification and to inform them of the nature of his vaccine approach, which utilized appropriate pustulate and not “putrified” material. Indeed, by the time Jenner had published his 3rd study on smallpox vaccination, upwards of 6,000 subjects had been vaccinated, many of whom were also tested for protective immunity. According to Jenner, “… the far greater part of them have since been inoculated with that of smallpox, and exposed to its infection in every rational way that could be devised, without effect” (Jenner, 1800). This body of work is similar to a modern Phase III clinical trial in both size and scope and undoubtedly played a role in the eventual use of this radical new approach to preventative medicine. In addition to protective efficacy, it was also important that Jenner demonstrated that arm-to-arm transmission between humans was possible, so that vaccine production was not reliant on sporadic outbreaks in cows (Plotkin and Plotkin, 2008). Although this provided initial feasibility at small scale, for the global eradication effort, further technical and safety refinements were made, including the development of vaccine production based on glycerinated calves’ lymph (Plotkin and Plotkin, 2008). By scarifying calves and harvesting/lyophilizing the virus-infected lymph for vaccine use, both safety and feasibility were greatly improved. In addition, because millions of doses of vaccine could be mass-produced at low cost, this further increased the feasibility of global use of the smallpox vaccine. In the end, this resulted in the final eradication of smallpox by 1980 (Fenner et al., 1988).
Figure 1. Contribution of Efficacy, Safety, Feasibility, and Cost to the development of vaccines.
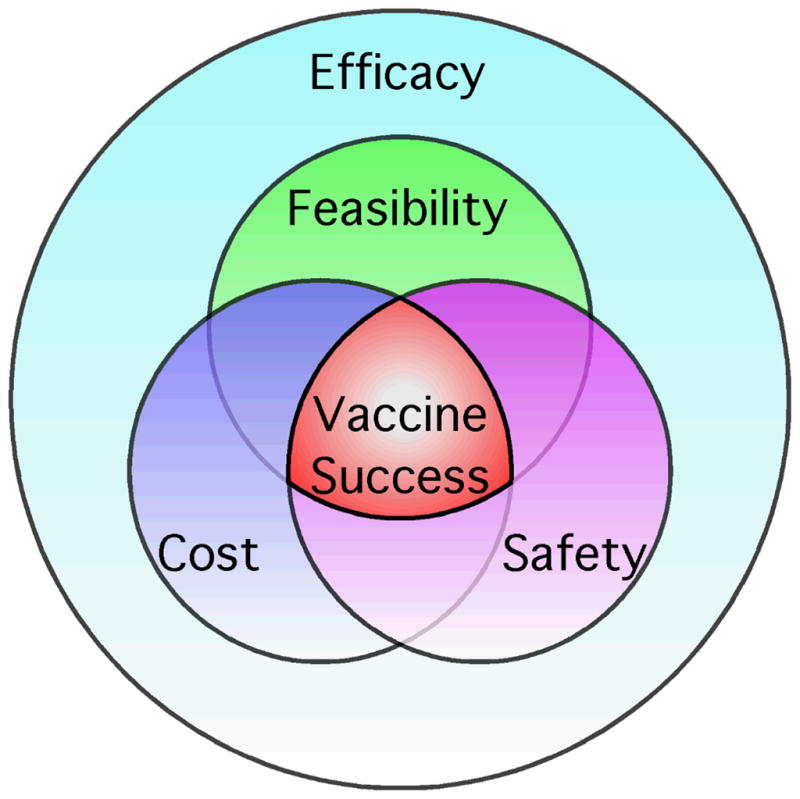
The development of a new vaccine is not based on protective efficacy alone. In order for a vaccine to be highly successful, it will need to meet minimum standards for efficacy, safety, feasibility, and cost. These are each important and inter-related considerations in the vaccine development process and only through the combined efforts of the manufacturer, regulatory authorities, and public health administrators, will a vaccine against a particular pathogen be fully utilized (Plotkin, 2005).
The current timeline for taking a vaccine from concept to licensed product is estimated at 10–15 years, though some licensed vaccines have taken up to 30 years (Douglas, Sadoff, and Samant, 2008). This extended timeline is due largely to the stringent pre-clinical and clinical testing that is required of human vaccine candidates. Vaccine development can also be expensive – with estimates approaching $500 to $800 million to bring a vaccine to market (Douglas, Sadoff, and Samant, 2008). Over time, advanced manufacturing techniques have reduced the costs of production but improvements in vaccine safety have resulted in increased costs as well. Vaccine safety is of growing importance because public fear of vaccination can dramatically reduce vaccine uptake and coverage, resulting in reduced herd immunity and in some cases, re-emergence of outbreaks of vaccine-preventable diseases such as measles (Amanna and Slifka, 2005; Jansen et al., 2003). To combat this trend, there is a push for higher vaccine purity, higher sterility assurance levels, reduction of animal-origin additives, enhanced testing for adventitious agents, and removal of preservatives – which has come with an associated increase in the usage of single dose containers (Gomez, Robinson, and Rogalewicz, 2008). Although these steps improve safety, this does not come without a cost. Indeed, the simple step of switching from multi-dose vials to single-dose vials can itself increase manufacturing costs by 250% (Lee et al., 2010). The ability of vaccine manufacturers to respond to updated regulatory requirements as well as unfounded or misguided fears of vaccines may have a significant impact on the development, availability, and cost of new vaccines in the future.
Persisting antibody following vaccination; the first line of defense against infection
In the past, the development of a potent antibody response has been the hallmark of a good vaccine and is the probable mechanism underlying vaccine efficacy for many of today’s most successful vaccines (Plotkin, 2010; Siegrist, 2008). Several vaccines have defined levels of serum antibody (as measured by ELISA, hemagglutination, or neutralization assays) that serve as correlates or surrogates of protective immunity (Table 1) (Plotkin, 2010). The smallpox vaccine was developed prior to the development of immunological tools but visual inspection of a “take” or Jennerian vesicle at the vaccination site served as a surrogate of protection. Field studies performed in the 1970’s have since indicated that a neutralizing titer of 1:20 (Sarkar, Mitra, and Mukherjee, 1975) to 1:32 (Mack, Noble, and Thomas, 1972) are likely to be sufficient to provide protective immunity against smallpox. The yellow fever vaccine was developed in the 1930’s (Monath, Cetron, and Teuwen, 2008) and, similar to the smallpox vaccine, there were no immunological assays available at that time that could be used to determine a correlate of immunity. However, in 1973 a series of experiments performed in rhesus macaques given varying doses of the yellow fever vaccine provided evidence that protection against lethal yellow fever challenge occurred in the majority of animals with neutralizing antibody titers of ≥0.7 LNI (log neutralizing index) (Mason et al., 1973). For measles, antiviral antibody levels of 120–200 mIU/mL are considered protective. A retrospective epidemiological study of a measles outbreak that occurred in a dormitory setting shortly following a blood drive provided the opportunity to determine the levels of pre-existing anti-measles antibody that were needed to protect students from overt disease (≥200 mIU/mL) (Chen et al., 1990). In an independent study, antibody levels measured within 10 days of exposure to measles and children with neutralizing titers above 125 mIU/mL were protected against clinical measles (Samb et al., 1995). Memory T cells and memory B cells were not examined in these studies and it is uncertain what contributions were made by these cell populations. However, review of vaccine-mediated immunity against smallpox and yellow fever indicate that pre-existing antibody plays the predominant role in protection (Amanna, Messaoudi, and Slifka, 2008).
Plasma cell contributions to vaccine-mediated immunity
The mechanisms that underpin that long-term antibody responses remain controversial (Amanna and Slifka, 2010; Elgueta, de Vries, and Noelle, 2010; Radbruch et al., 2006). Terminally differentiated plasma cells (PC) are the primary source of serum antibody and antibody secreting cells may produce up to 20,000 molecules of immunoglobulin per second (Conrad and Ingraham, 1974; Helmreich, Kern, and Eisen, 1961; Helmreich, Kern, and Eisen, 1962). With the ability to block virus at the site of infection or prevent/reduce systemic spread, pre-existing antibody plays a clear role in vaccine-mediated defense against infection. At the mucosal surfaces, where many pathogens gain entry to the host, IgA plays a significant protective role. Mucosal IgA is generated by local PC and released as dimeric IgA (dIgA). After release from the PC, dIgA can bind the polymeric immunoglobulin receptor (pIgR) at the basolateral surface of mucosal epithelial cells. This complex is actively transported through the epithelial cell, cleaved to form fully mature secretory IgA (SIgA), and then excreted into the mucosa. Several vaccines, such as those targeting cholera and typhoid fever, have been developed specifically to promote SIgA responses through oral administration, but immunity appears to wane shortly after vaccination (Holmgren and Czerkinsky, 2005).
By contrast, parenterally administered vaccines often elicit long-lived serum IgG responses, but limited SIgA responses. IgG is maintained at approximately 10 mg/mL in the circulation and is important in combating systemic viral infections. Indeed, passive immunotherapy has been used for decades to treat agammaglobulinemia as well as a number of infectious diseases (Amanna and Slifka, 2009; Casadevall, 1999; Eibl, 2008). In addition, through the process of transudation, IgG may also play an important role at mucosal sites. While the active transfer of SIgA across epithelial barriers is well described, the mechanism for IgG transudation is often described as passive diffusion, though active transport has been reported (Spiekermann et al., 2002). Nevertheless, transudation of high-titer serum IgG is thought to have a significant protective role against several viral pathogens. For example, vaccines against human papilloma virus (HPV), an infectious agent of global importance, may protect primarily through the transudation of serum IgG at the cervical mucosa (Schwarz and Leo, 2008). In one preliminary study with a relatively small number of subjects, investigators found that non-ovulating women had a positive correlation between cervical antibody titers and serum antibody titers (r = 0.86) whereas ovulating women did not (r = 0.27) (Nardelli-Haefliger et al., 2003). In the genital tract, transudation is believed to occur through a process of mass transfer, where an imbalance of hydrostatic pressures force the serum IgG across the blood capillary walls into the cervical mucus (Schwarz and Leo, 2008). Similar mechanisms of transudation also play a role in protecting against respiratory infections. Respiratory syncytial virus (RSV), is a pathogen of significant concern in infants and children that has eluded the development of a successful vaccine (Blanco et al., 2010). However, one of the most effective preventative strategies for at-risk infants is the parenteral administration of a highly neutralizing IgG monoclonal antibody (Empey, Peebles, and Kolls, 2010; Krilov et al., 2009). The use of parenterally administered IgG for the specific neutralization of a respiratory pathogen again points to the ability of systemic IgG to play an important role at mucosal surfaces, especially those in which severe disease is associated with infection of the lower respiratory tract. Given the importance that systemic antibody responses have in protection against viral infection, a key goal for vaccine development is to induce effective, long-term antibody responses. In order to achieve this goal, it is important to understand the mechanisms involved with generating persistent antibody responses and immunological memory.
The theories underlying PC survival (and therefore antibody maintenance) fall into two general categories (Amanna and Slifka, 2010). One theory is that long-lived antibody responses are memory B cell (MBC)-dependent and that MBC are required to undergo a certain degree of proliferation resulting in antibody-producing daughter cells which home to the BM, replenish declining PC numbers and maintain steady-state levels of serum antibody (Bernasconi, Traggiai, and Lanzavecchia, 2002; Traggiai, Puzone, and Lanzavecchia, 2003). An alternative theory is that long-lived antibody responses are MBC-independent and that plasma cells can be intrinsically long-lived and able to sustain protective antibody responses for long periods of time without the need for replenishment (Amanna and Slifka, 2010; Elgueta, de Vries, and Noelle, 2010; Radbruch et al., 2006; Slifka and Ahmed, 1998). Tritium incorporation studies in rats indicate that PC (or their immediate precursors) can be long-lived (Miller, 1964). Likewise, using modern techniques such as BrdU incorporation, PC have also been shown to be long-lived with little turn-over (Manz, Thiel, and Radbruch, 1997). One caveat is that a MBC could theoretically differentiate into a PC without undergoing cell division. This may be unlikely since several studies have shown that antibody responses are relatively resistant to MBC depletion (Ahuja et al., 2008; DiLillo et al., 2008; Slifka et al., 1998). Similarly, limited observational studies in human subjects treated with anti-CD20 antibodies indicate that vaccine-mediated antibody responses against tetanus remain largely unaltered for at least one year following depletion of peripheral CD20+ B cells (Cambridge et al., 2003). Taken together, these lines of evidence indicate that long-lived PC are the cell type responsible for maintaining long-term vaccine-induced antibody responses.
Despite a central role in vaccine-mediated immunity, the molecular mechanisms that drive PC development and allow for prolonged survival remain largely unknown. A subpopulation of long-lived PC remain in the spleen and/or draining lymph nodes following infection or vaccination, but the majority of PC occupy survival niches within the bone marrow (Benner, Hijmans, and Haaijman, 1981; Hyland et al., 1994; Slifka, Matloubian, and Ahmed, 1995; Tokoyoda et al., 2009). It is assumed that chemotactic factors allow plasma cells to migrate to the BM, and once there, local adhesion and/or chemokine factors maintain survival (Tokoyoda et al., 2009). However, given the complexity and possible redundancy of chemokine signaling, it may be difficult to determine the key factors involved in this process. For instance, while the CXCL12-CXCR4 interaction has been shown to be important in the early migration of PC to the BM (Hargreaves et al., 2001; Hauser et al., 2002), it seems to be dispensable for long-term antibody responses (Nie et al., 2004). Similarly, although IL-6 may be important for in vitro survival of isolated PC (Cassese et al., 2003), IL-6-deficient mice demonstrate no long-term humoral defects following immunization with the prototypical T cell-dependent antigen, ovalbumin (Cassese et al., 2003). In contrast, in vivo blockade of the adhesion molecules, LFA-1 (lymphocyte-associated antigen-1) and VLA-4 (integrin α4β1, very late antigen-4) leads to the rapid loss of a previously established antigen-specific antibody response (DiLillo et al., 2008). PC survival signals provided through the TNF family members, BAFF (B cell-activating factor of the TNF family) and APRIL (a proliferation-inducing ligand), have recently gained attention in PC maintenance. Both BAFF (also known as BlyS) and APRIL can bind the TNF receptors, TACI (transmembrane activator and calcium modulator ligand interactor) and BCMA (B cell maturation antigen), with only BAFF binding to the BAFF receptor (BAFF-R) (Mackay and Schneider, 2009). This system has been well described in peripheral B cell survival and development, but until recently, the role of BAFF and APRIL (and their associated receptors) in plasma cell development remained unclear. The first demonstration that this system might also affect terminal B cell development came from analysis of BCMA knockout mice (O’Connor et al., 2004). These animals demonstrated normal induction of TD antibody responses but showed decreased maintenance of PC. These details, along with the observation that BCMA surface receptor expression in humans appears limited to CD138+ B cells (Darce et al., 2007), points to the BAFF/APRIL:BCMA interactions as a key survival mechanism for PC. Further information to support this hypothesis was recently demonstrated through blockade of in vivo signaling by administration of soluble versions of BAFF-R (blocks only BAFF) and TACI (blocks both BAFF and APRIL) (Benson et al., 2008). The concurrent blockade of BAFF and APRIL led to a significant drop in antigen-specific plasma cells, 3 months after the establishment of a TD immune response. Intriguingly, while this blockade decreased the number of established PC, MBC populations appeared resistant to treatment, again pointing to a divergence between these two B cell populations. Based on adoptive transfer studies with BAFF−/− or APRIL−/− deficient mice, APRIL appears to have a predominant role over BAFF in supporting long-lived PC development and maintenance (Belnoue et al., 2008). Blimp-1 (B-lymphocyte induced maturation protein-1) expression is critical for both the development and maintenance of plasma cells. (Kallies and Nutt, 2007). Loss of Blimp-1 expression in conditional Blimp-1 knockout mice results in a significant drop in previously established TD antibody responses (Shapiro-Shelef et al., 2005). Deficiencies in the adaptor protein, SAP (SLAM-associated protein), allow the initial production of normal antibody titers, but long-term antibody maintenance is significantly impaired (Crotty et al., 2003b), possibly due to decreased contact time between B cells and T cells in the GC reaction (Qi et al., 2008). Further investigation into the key mechanisms that support PC survival in vivo, possibly through Blimp-1 and SAP expression, could be important in understanding the nature of long-term vaccine-mediated antibody responses.
The role of memory B cells in vaccine-mediated immunity
Unlike antibody-secreting plasma cells, vaccine-induced memory B cells do not provide direct protection against infection. Instead, they represent an important second line of immune defense that is initiated only if pre-existing antibody levels are too low to prevent infection or if the invading pathogen is able to circumvent the pre-existing antibody response (e.g., high dose exposure, antigenic mutation, etc.). Live-attenuated dengue virus vaccination provides an interesting case in point. To be effective, this vaccine requires tetravalent antibody responses against all 4 serotypes of dengue (Whitehead et al., 2007). Following primary vaccination, neutralizing antibody responses are typically directed to only a subset of the 4 serotypes but booster vaccination fails to elicit antibody responses to the remaining dengue serotypes unless vaccinations are spaced at least 4–6 months apart (Morrison et al., 2010; Whitehead et al., 2007). This indicates that pre-existing immunity in the form of circulating antibodies (and potentially virus-specific T cells) prevent infection by the vaccine strains of virus to the point that primary B cell responses and anamnestic responses by memory B cells are blunted or prevented altogether due to lack of sufficient viral replication. This pre-existing antibody-based phenomenon is not new; it has long been appreciated that high levels of maternal antibodies will hinder the induction of host immunity in infants, and this is especially problematic for replicating live-attenuated vaccines such as the MMR vaccine (Hayden, 1979). To overcome primary vaccine failure due to the protective effect of pre-existing maternally-derived antibodies, the timing of MMR vaccination is based primarily on two factors; 1) the earliest age at which high seroconversion rates can be consistently elicited and 2) the age group with the highest risk of severe disease (Orenstein et al., 1986; Strebel et al., 2008). Bearing these factors in mind, pre-existing antibody represents the initial protective barrier elicited by vaccine-induced immunity and memory B cell responses are invoked only if a given pathogen has breached this critical first line of defense.
Following vaccination or viral infection, memory B cell responses can be remarkably long-lived. For example, vaccine-induced B cell memory is maintained for decades after smallpox vaccination (Crotty et al., 2003a). This is not unique to this one vaccine since a number of other virus and vaccine antigens also induce long-lived memory B cell responses (Amanna, Carlson, and Slifka, 2007). Memory B cells maintain cell surface expression of membrane-bound immunoglobulins (which together with Igαand Igβform the B cell receptor; BcR), and B cells typically require crosslinking of the BcR in order to become fully activated to proliferate and differentiate into antibody-secreting daughter cells (Metzger, 1992; Pierce and Liu, 2010). In vitro, this can be accomplished by exposing B cells to staphylococcal protein G (which binds IgG molecules) in combination with toll-like receptor activation (Crotty et al., 2004). In addition, memory B cells can also be activated in vitro by combinations of TLR agonists and cytokines in the absence of BcR stimulation (Bernasconi, Traggiai, and Lanzavecchia, 2002), but in vivo, activation through the BcR is most likely to be triggered by re-infection and subsequent direct exposure to specific cognate antigen (Amanna and Slifka, 2010). The lack of continuous polyclonal activation and differentiation into antibody-secreting plasma cells may explain why B cell memory persists following hepatitis B vaccination despite the loss of detectable anti-HBsAg antibodies in the serum. Moreover, this may also explain why B cell memory is insufficient to protect against acute hepatitis B infection (as indicated by induction of hepatitis B core-specific antibodies), but appears to protect against progression to chronic hepatitis (Lin et al., 2003; Whittle et al., 1995; Young et al., 2003). Similarly, B cell memory is observed following vaccination with Haemophilus influenzae b (Hib) even after serum antibody responses have dropped below detection (Goldblatt et al., 1998) but pre-existing B cell memory may not necessarily prevent invasive Hib-associated disease (Lambert, Liu, and Siegrist, 2005; McVernon et al., 2003). This suggests that the role of memory B cells is not to block infection per se or to maintain serum antibody levels (Amanna and Slifka, 2010), but instead represents an independently regulated B cell population (Amanna, Carlson, and Slifka, 2007; Nanan et al., 2001) that is involved with eliciting anamnestic antibody responses and, in certain cases, reducing disease severity following infection. It is important to note that this role for memory B cells may be applicable only to pathogens that have a relatively long incubation period prior to disease progression, which allows for B cell activation, proliferation, differentiation, and de novo synthesis of antibody to occur in order to ameliorate ensuing pathology.
The contribution of memory T cells to vaccine-mediated protection
Virus-specific CD8+ T cells and CD4+ T cells represent the cellular arm of adaptive immunity triggered during infection or vaccination. Similar to memory B cells, memory T cells do not directly block infection, but they can reduce or eliminate viral replication after infection has occurred. For viruses that cause disease mainly through systemic spread (e.g., polio) or lower respiratory tract infection (e.g., influenza), protective immunity is typically attributed to pre-existing antigen-specific antibodies and pre-existing T cell memory may not be required to spare the individual from death or severe disease. This does not mean that T cells are unimportant – indeed, by reducing viral replication, vaccine-induced antibodies may function, at least in part, by suppressing viral growth until primary T cell responses are mounted. Not surprisingly, many of the first successful vaccines were developed against viruses that reside within this category of pathogens. However, for more complex viruses with a large number of serotypes (e.g., adenoviruses), rapid mutation rates (e.g., HIV) or the ability to form latency (e.g., EBV, CMV, HSV, VZV, HIV), the role of strong cellular immune responses in controlling and containing infection becomes increasingly important.
Memory T cells possess several key functional attributes that distinguish them from their naïve counterparts. Memory T cells can respond to lower amounts of peptide antigen, produce a wider array of inflammatory or antiviral cytokines, elicit rapid cytolytic activity, and home efficiently to either lymphoid or non-lymphoid compartments (Kaech, Wherry, and Ahmed, 2002; Lefrancois, 2006; Sallusto et al., 2010; Whitton et al., 2004). It was thought that memory T cells also proliferated more rapidly than naïve T cells following viral infection, but accumulating evidence (Stock et al., 2006; Whitmire, Eam, and Whitton, 2008) indicates that both CD4+ and CD8+ memory T cells undergo a 48–72 hour lag period that is similar to naïve T cells prior to initiating an antigen-specific proliferation program. Following this initial lag period, T cell proliferation in vivo is extremely rapid, reaching estimated cell division rates of once every 6–8 hours (Murali-Krishna et al., 1998), 4 hours (Lawrence and Braciale, 2004; Whitmire, Eam, and Whitton, 2008) or possibly as short as once every 2 hours (Yoon, Kim, and Braciale, 2010). There is much to be learned about the biological mechanisms that allow for such rapid cell division to occur in vivo. Antigen-specific T cells are known to “cannibalize” target cells by incorporating APC membranes into the responding T cell by a process of TcR-mediated endocytosis (Arnold, Davidian, and Mannie, 1997; Beadling and Slifka, 2006; Huang et al., 1999; Hudrisier and Bongrand, 2002; Hudrisier et al., 2001; Hwang et al., 2000; Joly and Hudrisier, 2003; Nepom, Benacerraf, and Germain, 1981; Patel et al., 1999; Rosenits et al., 2010; Tomaru et al., 2003; Tsang, Chai, and Lechler, 2003; Wetzel, McKeithan, and Parker, 2005). It would be interesting if the active acquisition of APC membranes during peptide-specific cell-to-cell interactions turns out to be an underlying mechanism that allows T cells to conserve metabolic energy while at the same time proliferating with extremely rapid doubling times during the critical early stages of infection.
Virus-specific T cell subsets were once broadly defined as activated “effector” T cells identified at or near the peak of an antiviral T cell response or they were defined as resting “memory” T cells following resolution of viral infection/viral clearance. Memory T cell populations have been further divided into “central memory” (TCM; CCR7+/CD62L+CD28+CD95+) or “effector memory” (TEM; CCR7−/CD62L−CD28−CD95+) subsets that differ by phenotype and function (Sallusto, Geginat, and Lanzavecchia, 2004; Sallusto et al., 1999). When initially defined, TCM were believed to reside in lymphoid tissues and lack immediate effector function, but were able to proliferate and different into TEM following challenge. In contrast, TEM reside primarily in non-lymphoid tissues and demonstrate more rapid effector functions (Sallusto et al., 1999). At least a subpopulation of TEM continue to be found in lymphoid tissues after resolution of viral infection, but interestingly, CD62L− TEM and CD62L+ TCM preferentially home to different anatomical sites within the spleen (Jung et al., 2010). Further studies have indicated that both TCM and TEM rapidly express effector functions including cytokine production and cytolytic activity (Wherry et al., 2003) and it is thought that their anatomical location is perhaps more important than the speed at which they become effectors (Sallusto et al., 2010).
In terms of vaccine-mediated protection, it is unclear if TCM or TEM are the most important subset for eliciting protection against viral challenge. In mice, virus-specific TCM are more protective than TEM when faced with respiratory (i.e., mucosal) vaccinia virus infection, acute systemic vaccinia virus infection, or chronic systemic LCMV infection (Wherry et al., 2003). On the other hand, vaccination of rhesus macaques with recombinant RhCMV vectors that induce primarily TEM T cell responses have been shown to provide substantial protection against mucosal SIV challenge (Hansen et al., 2009). One complication with these narrowly defined TCM or TEM subsets is that they are not homogeneous memory T cell populations. Each of these T cell populations can be further divided into more discrete T cell subsets with defined antiviral functions, phenotypic markers, and tissue homing capacity (Sallusto, Geginat, and Lanzavecchia, 2004). Indeed, there is evidence that other activation markers such as CD27 and CD43 may be superior to the TCM/TEM differentiation marker, CD62L, in predicting the recall responses of memory T cells upon viral challenge (Hikono et al., 2007). Likewise, the surface antigens, KLRG1 and CD127, have been used extensively to subdivide effector and memory T cell subsets and these phenotypic markers are useful for predicting whether the virus (or vaccine) induces primarily short-lived memory (KLRG1+ CD127-) or long-lived memory (KLRG1−CD127+) T cell responses (Cui and Kaech, 2010; Huster et al., 2004; Joshi et al., 2007; Kaech et al., 2003; Schluns et al., 2000). It is possible that different types of T cell memory will be required for different viral pathogens and this will most likely need to be determined on a case-by-case basis.
Cytotoxic CD4+ T cells – are they involved with protection following human vaccination?
There has been considerable emphasis placed on the induction and analysis of vaccine-induced CD8+ T cell responses. CD4+ T cell responses, on the other hand, are thought to be more important for development of effective humoral immune responses (i.e., T cell-dependent antibody responses) and the induction of effective CD8+ T cell responses, and the potential protective role for vaccine-induced CD4+ T cells during infection is not as well characterized. Cytotoxic MHC Class II-restricted CD4+ T cell responses have been identified in mice (Brown et al., 2009; Jellison, Kim, and Welsh, 2005) but in rodents, CD8+ CTL responses appear to be much more common. In contrast, there are several examples of virus-specific MHC Class II-restricted CD4+ CTL responses have been described in clinical studies (Bourgault et al., 1989; Demkowicz et al., 1996; Erickson and Walker, 1993; Littaua et al., 1992; Martorelli et al.; Mitra-Kaushik et al., 2007; Penna et al., 1992; Schmid, 1988; Walker and Slifka, 2010) as well as in chimpanzees (Zarling et al., 1987). Cytotoxic CD4+ T cells have been identified directly ex vivo following human measles virus infection (Jaye et al., 1998). Moreover, virus-specific CD4+ T cell memory can also be long-lived and following in vitro restimulation, cytotoxic CD4+ T cells have been identified for up to 50 years after smallpox vaccination (Demkowicz et al., 1996). Induction of cytolytic CD4+ T cell responses have been documented following infection with a number of viruses including measles (Jaye et al., 1998), vaccinia virus (Demkowicz et al., 1996; Erickson and Walker, 1993; Littaua et al., 1992; Mitra-Kaushik et al., 2007), polio (Wahid, Cannon, and Chow, 2005), dengue (Green et al., 1997), influenza (Bourgault et al., 1989), hepatitis B virus (Penna et al., 1992), varicella zoster virus (Arvin et al., 1991), Epstein Barr virus (Bourgault et al., 1989; Martorelli et al.; Munz et al., 2000), herpes simplex virus (Schmid, 1988) and CMV (Appay et al., 2002; Gyulai et al., 2000) as well as after vaccination with non-replicating antigens such as tetanus toxoid (Valmori et al., 1994). One caveat to the potential effectiveness of cytotoxic CD4+ T cell memory is that they would be limited to recognition of only a subset of host cells that express MHC Class II. However, this may not be an overriding limitation because many cell types including T cells (Miller et al., 2008) and respiratory epithelial cells (Hegde and Johnson, 2003; Papon et al., 2002; Rees et al., 2003; Rossi et al., 1990; Striz et al., 2000; Wang et al., 1997) have the ability to upregulate MHC Class II (e.g., HLA-DR) expression following activation. Bearing this in mind, it is possible that cytotoxic CD4+ T cells in humans and non-human primates may play a more substantial role in mucosal immunity than previously believed and this represents an important area that is worthy of further investigation.
Challenges in defining vaccine-mediated correlates of immunity to complex pathogens
Nearly all FDA-approved vaccines use seroconversion as the primary endpoint for determining vaccine immunogenicity. The only exception to this rule is the vaccinia virus-based smallpox vaccines, Dryvax and ACAM2000, in which the primary endpoint is development of a pustular lesion (i.e., a Jennerian vesicle) at the site of vaccination (Amanna, Messaoudi, and Slifka, 2008). It is important to note that the use of serum antibody titers as the primary endpoint does not necessarily mean that the protective immunity elicited by a particular vaccine is mediated by antibodies. In the past, serological analysis of vaccine immunogenicity was easier to perform, more amenable to high throughput analysis, and often easier to interpret than the analysis of vaccine-mediated T cell responses. This is especially true for vaccines that were developed and approved before the 1970’s when reagents for analyzing T cell responses did not even exist (Walker and Slifka, 2010).
For vaccines against single bacterial toxins (e.g., tetanus toxin) or invariant viruses (e.g., yellow fever virus), analysis of serum neutralizing antibody titers provides clear and definitive information on vaccine efficacy and protection. For instance, a tetanus toxin-specific neutralizing titer of ≥0.01 International Units/mL (Goulon et al., 1972; Wolters and Dehmel, 1942) or a yellow fever virus-specific log neutralizing index (LNI) of ≥0.7 (Mason et al., 1973) is indicative of protective immunity (Table 1) (Amanna, Messaoudi, and Slifka, 2008). Immunological analysis is facilitated by the use of standardized techniques such as neutralizing assays or enzyme-linked immunosorbant assays (ELISA) and for many vaccines, there are international serum standards that can be utilized to validate in-house analysis of serological immunity. For viruses such as HIV with a high mutation rate and a complex lifecycle, it has been difficult to develop a single serological assay that can be used for determining vaccine efficacy – and it is even more problematic to determine an antibody titer/humoral response that might be considered protective. There are a number of ways in which virus-specific antibodies can interfere with viral infection/replication/transmission (Burton, 2002; McElrath and Haynes, 2010). For this reason, there are >10 different assays for measuring HIV-specific antibody responses that vary in terms of complexity and the ability to be standardized and validated [(McElrath and Haynes, 2010); Table S1]. This is further complicated by questions regarding which strains of HIV or pseudovirus are most appropriate to use for determining antibody responses, etc. Development and distribution of an appropriate broadly neutralizing HIV-specific international serum standard may also facilitate the standardization of serological tests for upcoming HIV vaccine candidates.
Analysis of virus-specific CD4+ and CD8+ T cell responses and their contribution to vaccine-mediated immunity is more complex than the analysis of antiviral antibody responses. Because humans express different haplotypes, different MHC-restricted peptide epitopes or whole proteins/virus lysates must be used to evaluate virus-specific T cell responses. Are some T cell epitopes more protective than others? Should a single peptide-specific response be measured as an immunological endpoint or should multiple peptide epitopes be measured and the magnitude of the total virus-specific T cell response be determined? The breadth of the vaccine-mediated CD8+ T cell response to HIV may be an important consideration; human vaccine recipients in the failed Step trial mounted a median response to only 1–2 epitopes from Gag, Pol, and Nef whereas rhesus macaques given the same vaccine elicited T cell responses to an average of 12 epitopes and were protected against viral challenge (McElrath and Haynes, 2010; Wilson et al., 2009). One of the complications involved with studying vaccine-mediated T cell responses in the context of clinical trials, is that different groups often use different approaches and different controls when quantitating antiviral T cell memory. However, the state of the art is constantly improving and HIV research is leading the way. A number of HIV-specific T cell assays are in use and two assays, IFNγELISPOT and intracellular cytokine staining by flow cytometry, are among the first to be validated (McElrath and Haynes, 2010). Having standardized/validated techniques is the first step necessary to provide unbiased comparisons between different studies or by different research organizations and this will likely expedite future vaccine evaluation.
For varicella zoster virus (VZV), an antibody titer of 5≥ gpELISA units is considered protective (Li et al., 2002), but this serological endpoint may not be reflective of the underlying vaccine-mediated T cell response, especially at later time points following vaccination (Amanna, Messaoudi, and Slifka, 2008). Indeed, this may be an example in which antibody is a surrogate or a co-correlate of immunity with the relative contributions by antiviral T cells being difficult to quantify in isolation. The major immunodominant peptide epitopes of VZV have not been mapped, so cellular immunity against this virus has been measured primarily by proliferation assays and skin tests in addition to ELISPOT assays (Gershon, Takahashi, and Seward, 2008). It is postulated that vaccines that induce CD8+ T cells with a multifunctional phenotype capable of producing IFNγ, TNFα, and IL-2 (among others) will be among the best vaccine candidates. However, development of a relatively high frequency of vaccine-induced multifunctional HIV-specific CD8+ T cells during the Step trial has been disappointing because there appeared to be no difference in pre-existing T cell memory among patients who became HIV-infected and their vaccinated controls (McElrath and Haynes, 2010). Many new vaccines against chronic viral pathogens are in various stages of development and the use of standardized T cell assays (if feasible) will be important in determining the cellular correlates of antiviral immunity.
Conclusions
Vaccine-mediated immunity is often multifactorial and the best protection is likely to be elicited by the combination of strong humoral and cell-mediated immune responses. Pre-existing virus-specific antibody represents the first line of defense against infection. However, if a viral pathogen is able to overcome vaccine-induced antibody responses and a productive infection occurs, then memory T cells and memory B cells are mobilized to initiate an anamnestic program of rapid proliferation and differentiation into effector cells. Protective immunity provided by the most successful vaccines will likely be a combination of residual persisting antibody, production of increased local and systemic levels of antibody by memory B cells, and active immunosurveillance for infected cells by rapidly proliferating virus-specific T cells. Defining the immunological correlates of immunity has been relatively straightforward for invariant viral pathogens in which antibody plays a predominant role in protection. This has been accomplished through field trials (e.g., smallpox), epidemiological studies (e.g., measles), or animal models (e.g., yellow fever). For more complex pathogens with complicated lifecycles or rapid mutation rates, the immunological correlates of protection may not be known and are likely to include a combination of vaccine-induced cellular and humoral immune responses. The challenge to virologists, immunologists, and vaccinologists will be to determine how to incorporate different levels of cellular and humoral immunity into a working model or algorithm of inter-related co-correlates that can be used reliably as a quantitative measure of protective immunity. Antiviral T cell and B cell responses do not act in isolation and although either arm of the vaccine-induced immune response may play a predominant role in protective immunity to a particular virus, they clearly function best together to efficiently protect against disease.
Acknowledgments
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health grants, R43 AI079898 (to I.J.A. and M.K.S.), UO1 AI082196 U54 (to M.K.S.), U54 AI081680 (to M.K.S.), and ONPRC grant RR00163 (to M.K.S.). Oregon Health and Science University (OHSU), I.J.A., and M.K.S. have a financial interest in Najít Technologies, Inc., a company that may have a commercial interest in the results of this research and technology.
Footnotes
These potential conflicts of interest have been reviewed and managed by OHSU and the Integrity Program Oversight Council.
References
- Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008;105(12):4802–7. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna I, Slifka MK. Public fear of vaccination: separating fact from fiction. Viral Immunol. 2005;18(2):307–15. doi: 10.1089/vim.2005.18.307. [DOI] [PubMed] [Google Scholar]
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: How is it defined? Hum Vaccin. 2008;4(4) doi: 10.4161/hv.4.4.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna IJ, Slifka MK. Wanted, dead or alive: new viral vaccines. Antiviral Res. 2009;84(2):119–30. doi: 10.1016/j.antiviral.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–38. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168(11):5954–8. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- Arnold PY, Davidian DK, Mannie MD. Antigen presentation by T cells: T cell receptor ligation promotes antigen acquisition from professional antigen-presenting cells. Eur J Immunol. 1997;27(12):3198–205. doi: 10.1002/eji.1830271217. [DOI] [PubMed] [Google Scholar]
- Arvin AM, Sharp M, Smith S, Koropchak CM, Diaz PS, Kinchington P, Ruyechan W, Hay J. Equivalent recognition of a varicella-zoster virus immediate early protein (IE62) and glycoprotein I by cytotoxic T lymphocytes of either CD4+ or CD8+ phenotype. J Immunol. 1991;146(1):257–64. [PubMed] [Google Scholar]
- Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C, Slifka MK. Quantifying viable virus-specific T cells without a priori knowledge of fine epitope specificity. Nat Med. 2006;12(10):1208–12. doi: 10.1038/nm1413. [DOI] [PubMed] [Google Scholar]
- Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111(5):2755–64. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- Benner R, Hijmans W, Haaijman JJ. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981;46(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180(6):3655–9. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601):2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- Blanco JC, Boukhvalova MS, Shirey KA, Prince GA, Vogel SN. New insights for development of a safe and protective RSV vaccine. Hum Vaccin. 2010;6(6):482–92. doi: 10.4161/hv.6.6.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgault I, Gomez A, Gomard E, Picard F, Levy JP. A virus-specific CD4+ cell-mediated cytolytic activity revealed by CD8+ cell elimination regularly develops in uncloned human antiviral cell lines. J Immunol. 1989;142(1):252–6. [PubMed] [Google Scholar]
- Brown DM, Kamperschroer C, Dilzer AM, Roberts DM, Swain SL. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol. 2009;257(1–2):69–79. doi: 10.1016/j.cellimm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR. Opinion: Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2(9):706–13. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, Webster AD. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48(8):2146–54. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- Casadevall A. Passive antibody therapies: progress and continuing challenges. Clin Immunol. 1999;93 (1):5–15. doi: 10.1006/clim.1999.4768. [DOI] [PubMed] [Google Scholar]
- Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171(4):1684–90. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- CDC. Advances in global measles control and elimination: summary of the 1997 international meeting. MMWR Recomm Rep. 1998;47(RR-11):1–23. [PubMed] [Google Scholar]
- Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162(5):1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- Conrad RE, Ingraham JS. Rate of hemolytic antibody production by single cells in vivo in rabbits. J Immunol. 1974;112(1):17–25. [PubMed] [Google Scholar]
- Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286(1–2):111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting Edge: Long-Term B Cell Memory in Humans after Smallpox Vaccination. J Immunol. 2003a;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003b;421(6920):282–7. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–66. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179(11):7276–86. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Hammarlund E, Slifka MK, Fruh K. Cowpox virus evades CTL recognition and inhibits the intracellular transport of MHC class I molecules. J Immunol. 2007;178(3):1654–61. doi: 10.4049/jimmunol.178.3.1654. [DOI] [PubMed] [Google Scholar]
- Demkowicz WEJ, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180(1):361–71. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- Douglas RG, Sadoff JC, Samant V. The vaccine industry. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 37–44. [Google Scholar]
- Dowdle WR, Coleman MT, Mostow SR, Kaye HS, Schoenbaum SC. Inactivated influenza vaccines. 2. Laboratory indices of protection. Postgrad Med J. 1973;49(569):159–63. doi: 10.1136/pgmj.49.569.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl MM. History of immunoglobulin replacement. Immunol Allergy Clin North Am. 2008;28(4):737–64. viii. doi: 10.1016/j.iac.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Elgueta R, de Vries VC, Noelle RJ. The immortality of humoral immunity. Immunol Rev. 2010;236:139–50. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- Empey KM, Peebles RS, Jr, Kolls JK. Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin Infect Dis. 2010;50(9):1258–67. doi: 10.1086/651603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson AL, Walker CM. Class I major histocompatibility complex-restricted cytotoxic T cell responses to vaccinia virus in humans. J Gen Virol. 1993;74(Pt 4):751–4. doi: 10.1099/0022-1317-74-4-751. [DOI] [PubMed] [Google Scholar]
- FDA. Complete List of Vaccines Licensed for Immunization and Distribution in the US. 2010 http://www.fda.gov/BiologicsBloodVaccines/Vaccies/ApprovedProducts/UCM093833.
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. The pathogenesis, immunology, and pathology of smallpox and vaccinia. World Health Organization; Geneva: 1988. Smallpox and its eradication; p. 1469. [Google Scholar]
- Fine PEM, Mulholland K. Community Immunity. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 17–36. [Google Scholar]
- Fiore AE, Finestone SM, Bell BP. Hepatitis A vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 177–203. [Google Scholar]
- Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24(15):2718–31. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Takahashi M, Seward JF. Varicella vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 915–958. [Google Scholar]
- Goldblatt D, Miller E, McCloskey N, Cartwright K. Immunological response to conjugate vaccines in infants: follow up study. BMJ. 1998;316(7144):1570–1. doi: 10.1136/bmj.316.7144.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez PL, Robinson JM, Rogalewicz J. Vaccine manufacturing. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 45–58. [Google Scholar]
- Goulon M, Girard O, Grosbuis S, Desormeau JP, Capponi MF. Antitetanus antibodies. Assay before anatoxinotherapy in 64 tetanus patients. Nouv Presse Med. 1972;1(45):3049–50. [PubMed] [Google Scholar]
- Green S, Kurane I, Pincus S, Paoletti E, Ennis FA. Recognition of dengue virus NS1-NS2a proteins by human CD4+ cytotoxic T lymphocyte clones. Virology. 1997;234(2):383–6. doi: 10.1006/viro.1997.8648. [DOI] [PubMed] [Google Scholar]
- Gyulai Z, Endresz V, Burian K, Pincus S, Toldy J, Cox WI, Meric C, Plotkin S, Gonczol E, Berencsi K. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J Infect Dis. 2000;181(5):1537–46. doi: 10.1086/315445. [DOI] [PubMed] [Google Scholar]
- Hall-Baker PA, Enrique Nieves J, Jajosky RA, Adams DA, Sharp P, Anderson WJ, Aponte JJ, Aranas AE, Katz SB, Mayes M, Wodajo MS, Onweh DH, Baillie J, Park M. Summary of Notifiable Diseases --- United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;57:1–93. [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194(1):45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, Manz RA. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169(3):1277–82. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- Hayden GF. Measles vaccine failure. A survey of causes and means of prevention. Clin Pediatr (Phila) 1979;18(3):155–6. 161–3, 167. doi: 10.1177/000992287901800308. [DOI] [PubMed] [Google Scholar]
- Hegde NR, Johnson DC. Human cytomegalovirus US2 causes similar effects on both major histocompatibility complex class I and II proteins in epithelial and glial cells. J Virol. 2003;77(17):9287–94. doi: 10.1128/JVI.77.17.9287-9294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmreich E, Kern M, Eisen HN. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961;236:464–473. [PubMed] [Google Scholar]
- Helmreich E, Kern M, Eisen HN. Observations on the mechanism of secretion of g-globulins by isolated lymph node cells. J Biol Chem. 1962;237(6):1925–1931. [PubMed] [Google Scholar]
- Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204(7):1625–36. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23(45):5205–11. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286(5441):952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- Hudrisier D, Bongrand P. Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. Faseb J. 2002;16(6):477–86. doi: 10.1096/fj.01-0933rev. [DOI] [PubMed] [Google Scholar]
- Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166(6):3645–9. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101(15):5610–5. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191(7):1137–48. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland L, Sangster M, Sealy R, Coleclough C. Respiratory virus infection of mice provokes a permanent humoral immune response. J Virol. 1994;68:6083–86. doi: 10.1128/jvi.68.9.6083-6086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179(2):489–92. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- Jansen VA, Stollenwerk N, Jensen HJ, Ramsay ME, Edmunds WJ, Rhodes CJ. Measles outbreaks in a population with declining vaccine uptake. Science. 2003;301(5634):804. doi: 10.1126/science.1086726. [DOI] [PubMed] [Google Scholar]
- Jaye A, Magnusen AF, Sadiq AD, Corrah T, Whittle HC. Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. J Clin Invest. 1998;102(11):1969–77. doi: 10.1172/JCI3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellison ER, Kim SK, Welsh RM. Cutting Edge: MHC Class II-Restricted Killing In Vivo during Viral Infection. J Immunol. 2005;174(2):614–8. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- Jenner E. An inquiry into the causes and effects of the variolae vaccinae. Sampson Low; London: 1798. [Google Scholar]
- Jenner E. A continuation of facts and observations relative to the variolae vaccinae, or cowpox. Sampson Low; London: 1800. [Google Scholar]
- Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4(9):815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol. 2010;185(9):5315–25. doi: 10.4049/jimmunol.1001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nature Rev Immunol. 2002;2(4):251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Kallies A, Nutt SL. Terminal differentiation of lymphocytes depends on Blimp-1. Curr Opin Immunol. 2007;19(2):156–62. doi: 10.1016/j.coi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin Exp Immunol. 1997;110(3):358–61. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krilov LR, Weiner LB, Yogev R, Fergie J, Katz BZ, Henrickson KJ, Welliver RC., Sr The 2009 COID recommendations for RSV prophylaxis: issues of efficacy, cost, and evidence-based medicine. Pediatrics. 2009;124(6):1682–4. doi: 10.1542/peds.2009-2681. [DOI] [PubMed] [Google Scholar]
- Lambert PH, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med. 2005;11(4 Suppl):S54–62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173(2):1209–18. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- Lee BY, Norman BA, Assi TM, Chen SI, Bailey RR, Rajgopal J, Brown ST, Wiringa AE, Burke DS. Single versus multi-dose vaccine vials: an economic computational model. Vaccine. 2010;28(32):5292–300. doi: 10.1016/j.vaccine.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- Li S, Chan IS, Matthews H, Heyse JF, Chan CY, Kuter BJ, Kaplan KM, Vessey SJ, Sadoff JC. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr Infect Dis J. 2002;21(4):337–42. doi: 10.1097/00006454-200204000-00014. [DOI] [PubMed] [Google Scholar]
- Lin YC, Chang MH, Ni YH, Hsu HY, Chen DS. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J Infect Dis. 2003;187(1):134–8. doi: 10.1086/345871. [DOI] [PubMed] [Google Scholar]
- Littaua RA, Takeda A, Cruz J, Ennis FA. Vaccinia virus-specific human CD4+ cytotoxic T-lymphocyte clones. J Virol. 1992;66(4):2274–80. doi: 10.1128/jvi.66.4.2274-2280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TM, Noble J, Jr, Thomas DB. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972;21(2):214–8. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9(7):491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388(10):133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- Martorelli D, Muraro E, Merlo A, Turrini R, Rosato A, Dolcetti R. Role of CD4+ cytotoxic T lymphocytes in the control of viral diseases and cancer. Int Rev Immunol. 2010;29(4):371–402. doi: 10.3109/08830185.2010.489658. [DOI] [PubMed] [Google Scholar]
- Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol. 1973;25(4):538–44. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter L, Kogelschatz K, Germann D. Serum levels of rubella virus antibodies indicating immunity: response to vaccination of subjects with low or undetectable antibody concentrations. J Infect Dis. 1997;175(4):749–55. doi: 10.1086/513967. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33(4):542–54. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVernon J, Johnson PD, Pollard AJ, Slack MP, Moxon ER. Immunologic memory in Haemophilus influenzae type b conjugate vaccine failure. Arch Dis Child. 2003;88(5):379–83. doi: 10.1136/adc.88.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H. Transmembrane signaling: the joy of aggregation. J Immunol. 1992;149(5):1477–87. [PubMed] [Google Scholar]
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Miller JJ. An autoradiographic study of plasma cell and lymphocyte survival in rat popliteal lymph nodes. J Immunol. 1964;92:673–681. [PubMed] [Google Scholar]
- Mitra-Kaushik S, Cruz J, Stern LJ, Ennis FA, Terajima M. Human cytotoxic CD4+ T cells recognize HLA-DR1-restricted epitopes on vaccinia virus proteins A24R and D1R conserved among poxviruses. J Immunol. 2007;179(2):1303–12. doi: 10.4049/jimmunol.179.2.1303. [DOI] [PubMed] [Google Scholar]
- Monath TP, Cetron MS, Teuwen DE. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; 2008. p. 1725. [Google Scholar]
- Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis. 2010;201(3):370–7. doi: 10.1086/649916. [DOI] [PubMed] [Google Scholar]
- Munz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, Zhang D, O’Donnell M, Steinman RM. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191(10):1649–60. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJD, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: A reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Nalin DR, Kuter BJ, Brown L, Patterson C, Calandra GB, Werzberger A, Shouval D, Ellerbeck E, Block SL, Bishop R, et al. Worldwide experience with the CR326F-derived inactivated hepatitis A virus vaccine in pediatric and adult populations: an overview. J Hepatol. 1993;18(Suppl 2):S51–5. doi: 10.1016/s0168-8278(05)80379-4. [DOI] [PubMed] [Google Scholar]
- Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2001;20(3–4):498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, De Grandi P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95(15):1128–37. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- Nepom JT, Benacerraf B, Germain RN. Acquisition of syngeneic I-A determinants by T cells proliferating in response to poly (Glu60Ala30Tyr10) J Immunol. 1981;127(3):888–92. [PubMed] [Google Scholar]
- Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200(9):1145–56. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199(1):91–8. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein WA, Markowitz L, Preblud SR, Hinman AR, Tomasi A, Bart KJ. Appropriate age for measles vaccination in the United States. Dev Biol Stand. 1986;65:13–21. [PubMed] [Google Scholar]
- Papon JF, Coste A, Gendron MC, Cordonnier C, Wingerstmann L, Peynegre R, Escudier E. HLA-DR and ICAM-1 expression and modulation in epithelial cells from nasal polyps. Laryngoscope. 2002;112(11):2067–75. doi: 10.1097/00005537-200211000-00030. [DOI] [PubMed] [Google Scholar]
- Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J Immunol. 1999;163(10):5201–10. [PubMed] [Google Scholar]
- Penna A, Fowler P, Bertoletti A, Guilhot S, Moss B, Margolskee RF, Cavalli A, Valli A, Fiaccadori F, Chisari FV, et al. Hepatitis B virus (HBV)-specific cytotoxic T-cell (CTL) response in humans: characterization of HLA class II-restricted CTLs that recognize endogenously synthesized HBV envelope antigens. J Virol. 1992;66(2):1193–8. doi: 10.1128/jvi.66.2.1193-1198.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SK, Liu W. The tipping points in the initiation of B cell signalling: how small changes make big differences. Nat Rev Immunol. 2010;10(11):767–77. doi: 10.1038/nri2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Why certain vaccines have been delayed or not developed at all. Health Aff (Millwood) 2005;24(3):631–4. doi: 10.1377/hlthaff.24.3.631. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA, Vidor E. Poliovirus vaccine - inactivated. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; Phildelphia, PA: 2008. pp. 605–630. [Google Scholar]
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 1–16. [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455(7214):764–9. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6(10):741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- Rees LE, Ayoub O, Haverson K, Birchall MA, Bailey M. Differential major histocompatibility complex class II locus expression on human laryngeal epithelium. Clin Exp Immunol. 2003;134(3):497–502. doi: 10.1111/j.1365-2249.2003.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenits K, Keppler SJ, Vucikuja S, Aichele P. T cells acquire cell surface determinants of APC via in vivo trogocytosis during viral infections. Eur J Immunol. 2010;40(12):3450–3457. doi: 10.1002/eji.201040743. [DOI] [PubMed] [Google Scholar]
- Rossi GA, Sacco O, Balbi B, Oddera S, Mattioni T, Corte G, Ravazzoni C, Allegra L. Human ciliated bronchial epithelial cells: expression of the HLA-DR antigens and of the HLA-DR alpha gene, modulation of the HLA-DR antigens by gamma-interferon and antigen-presenting function in the mixed leukocyte reaction. Am J Respir Cell Mol Biol. 1990;3(5):431–9. doi: 10.1165/ajrcmb/3.5.431. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–63. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Samb B, Aaby P, Whittle HC, Seck AM, Rahman S, Bennett J, Markowitz L, Simondon F. Serologic status and measles attack rates among vaccinated and unvaccinated children in rural Senegal. Pediatr Infect Dis J. 1995;14(3):203–9. doi: 10.1097/00006454-199503000-00007. [DOI] [PubMed] [Google Scholar]
- Sarkar JK, Mitra AC, Mukherjee MK. The minimum protective level of antibodies in smallpox. Bull World Health Organ. 1975;52(3):307–11. [PMC free article] [PubMed] [Google Scholar]
- Schiller JT, Frazer IH, Lowy DR. Human papillomavirus vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 243–258. [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Schmid DS. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4+, CD8- T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988;140(10):3610–6. [PubMed] [Google Scholar]
- Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol Oncol. 2008;110(3 Suppl 1):S1–10. doi: 10.1016/j.ygyno.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med. 2005;202(11):1471–6. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist C-A. Vaccine Immunology. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders/Elsevier; 2008. p. 1725. [Google Scholar]
- Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol. 1996;106(2):170–4. doi: 10.1093/ajcp/106.2.170. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10(3):252–8. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69(3):1895–902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196(3):303–10. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AT, Jones CM, Heath WR, Carbone FR. Cutting edge: central memory T cells do not show accelerated proliferation or tissue infiltration in response to localized herpes simplex virus-1 infection. J Immunol. 2006;177(3):1411–5. doi: 10.4049/jimmunol.177.3.1411. [DOI] [PubMed] [Google Scholar]
- Strebel PM, Papania MJ, Dayan GH, Halsey NA. Measles vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Saunders/Elsevier; Philadelphia, PA: 2008. pp. 353–398. [Google Scholar]
- Striz I, Mio T, Adachi Y, Carnevali S, Romberger DJ, Rennard SI. Effects of interferons alpha and gamma on cytokine production and phenotypic pattern of human bronchial epithelial cells. Int J Immunopharmacol. 2000;22(8):573–85. doi: 10.1016/s0192-0561(00)00020-5. [DOI] [PubMed] [Google Scholar]
- Tizard I. Grease, anthraxgate, and kennel cough: a revisionist history of early veterinary vaccines. Adv Vet Med. 1999;41:7–24. doi: 10.1016/s0065-3519(99)80005-6. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Chang HD, Radbruch A. Organization and maintenance of immunological memory by stroma niches. Eur J Immunol. 2009;39(8):2095–9. doi: 10.1002/eji.200939500. [DOI] [PubMed] [Google Scholar]
- Tomaru U, Yamano Y, Nagai M, Maric D, Kaumaya PT, Biddison W, Jacobson S. Detection of virus-specific T cells and CD8+ T-cell epitopes by acquisition of peptide-HLA-GFP complexes: analysis of T-cell phenotype and function in chronic viral infections. Nat Med. 2003;9(4):469–76. doi: 10.1038/nm845. [DOI] [PubMed] [Google Scholar]
- Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21(Suppl 2):S35–7. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101(7):2704–10. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- Tulman ER, Delhon G, Afonso CL, Lu Z, Zsak L, Sandybaev NT, Kerembekova UZ, Zaitsev VL, Kutish GF, Rock DL. Genome of horsepox virus. J Virol. 2006;80(18):9244–58. doi: 10.1128/JVI.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmori D, Sabbatini A, Lanzavecchia A, Corradin G, Matricardi PM. Functional analysis of two tetanus toxin universal T cell epitopes in their interaction with DR1101 and DR1104 alleles. J Immunol. 1994;152(6):2921–9. [PubMed] [Google Scholar]
- Wahid R, Cannon MJ, Chow M. Virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. J Virol. 2005;79(10):5988–95. doi: 10.1128/JVI.79.10.5988-5995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Slifka MK. Longevity of T-cell memory following acute viral infection. Adv Exp Med Biol. 2010;684:96–107. doi: 10.1007/978-1-4419-6451-9_8. [DOI] [PubMed] [Google Scholar]
- Wang D, Levasseur-Acker GM, Jankowski R, Kanny G, Moneret-Vautrin DA, Charron D, Lockhart A, Swierczewski E. HLA class II antigens and T lymphocytes in human nasal epithelial cells. Modulation of the HLA class II gene transcripts by gamma interferon. Clin Exp Allergy. 1997;27(3):306–14. [PubMed] [Google Scholar]
- Weibel RE, Buynak EB, McLean AA, Hilleman MR. Long-term follow-up for immunity after monovalent or combined live measles, mumps, and rubella virus vaccines. Pediatrics. 1975;56(3):380–7. [PubMed] [Google Scholar]
- Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schodel F, Morrison VA, Kauffman CA, Straus SE, Schmader KE, Davis LE, Levin MJ. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200(7):1068–77. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel SA, McKeithan TW, Parker DC. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J Immunol. 2005;174(1):80–9. doi: 10.4049/jimmunol.174.1.80. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, Von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- White CJ, Kuter BJ, Ngai A, Hildebrand CS, Isganitis KL, Patterson CM, Capra A, Miller WJ, Krah DL, Provost PJ, et al. Modified cases of chickenpox after varicella vaccination: correlation of protection with antibody response. Pediatr Infect Dis J. 1992;11(1):19–23. doi: 10.1097/00006454-199201000-00006. [DOI] [PubMed] [Google Scholar]
- Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5(7):518–28. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- Whitmire JK, Eam B, Whitton JL. Tentative T cells: memory cells are quick to respond, but slow to divide. PLoS Pathog. 2008;4(4):e1000041. doi: 10.1371/journal.ppat.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle HC, Maine N, Pilkington J, Mendy M, Fortuin M, Bunn J, Allison L, Howard C, Hall A. Long-term efficacy of continuing hepatitis B vaccination in infancy in two Gambian villages. Lancet. 1995;345(8957):1089–92. doi: 10.1016/s0140-6736(95)90822-6. [DOI] [PubMed] [Google Scholar]
- Whitton JL, Slifka MK, Liu F, Nussbaum AK, Whitmire JK. The regulation and maturation of antiviral immune responses. Adv Virus Res. 2004;63:181–238. doi: 10.1016/S0065-3527(04)63003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Rabies vaccines. WHO position paper. Wkly Epidemiol Rec. 2007;82(49–50):425–35. [PubMed] [Google Scholar]
- Wilson NA, Keele BF, Reed JS, Piaskowski SM, MacNair CE, Bett AJ, Liang X, Wang F, Thoryk E, Heidecker GJ, Citron MP, Huang L, Lin J, Vitelli S, Ahn CD, Kaizu M, Maness NJ, Reynolds MR, Friedrich TC, Loffredo JT, Rakasz EG, Erickson S, Allison DB, Piatak M, Jr, Lifson JD, Shiver JW, Casimiro DR, Shaw GM, Hahn BH, Watkins DI. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol. 2009;83(13):6508–21. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters KL, Dehmel H. Abschliessende untersuchungen uber die Tetanus Prophylaxe durch active Immunisierung. Zeitschrift fur Hyeitschrift. 1942;124:326–332. [Google Scholar]
- Yoon H, Kim TS, Braciale TJ. The cell cycle time of CD8 T cells responding in vivo is controlled by the type of antigenic stimulus. PLoS One. 2010;5(11):e15423. doi: 10.1371/journal.pone.0015423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BW, Lee SS, Lim WL, Yeoh EK. The long-term efficacy of plasma-derived hepatitis B vaccine in babies born to carrier mothers. J Viral Hepat. 2003;10(1):23–30. doi: 10.1046/j.1365-2893.2003.00386.x. [DOI] [PubMed] [Google Scholar]
- Zarling JM, Eichberg JW, Moran PA, McClure J, Sridhar P, Hu SL. Proliferative and cytotoxic T cells to AIDS virus glycoproteins in chimpanzees immunized with a recombinant vaccinia virus expressing AIDS virus envelope glycoproteins. J Immunol. 1987;139(4):988–90. [PubMed] [Google Scholar]


