Abstract
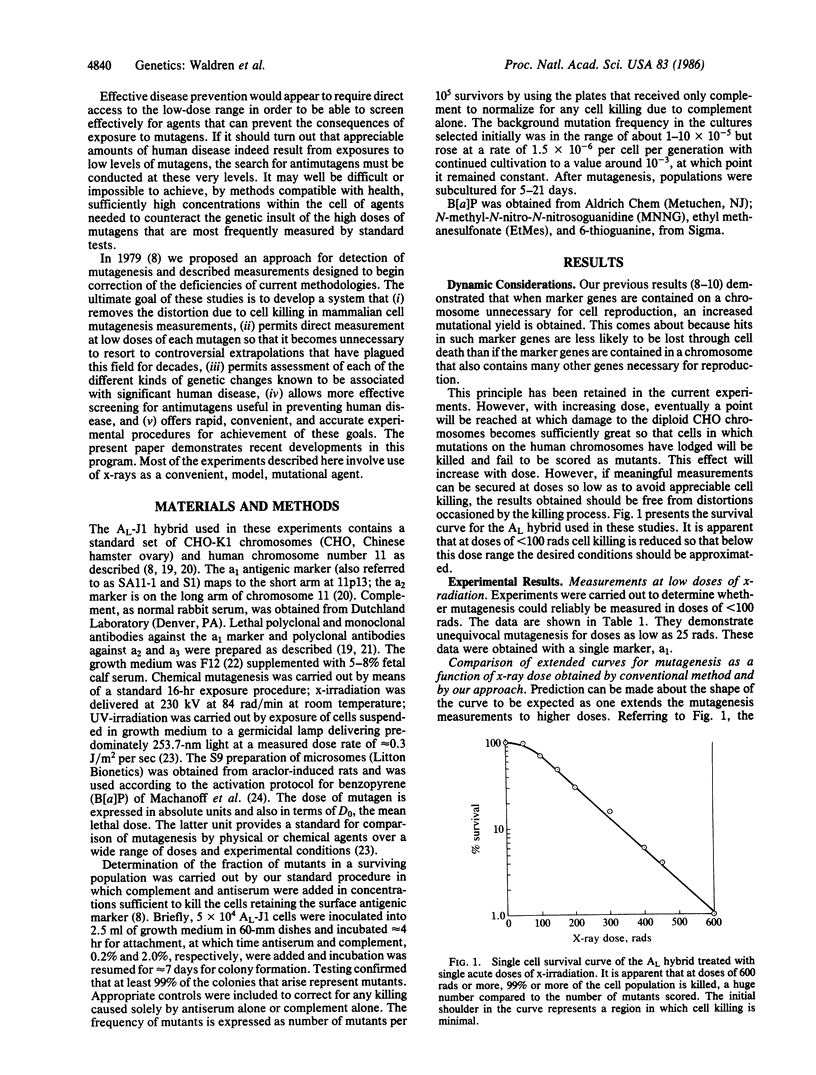
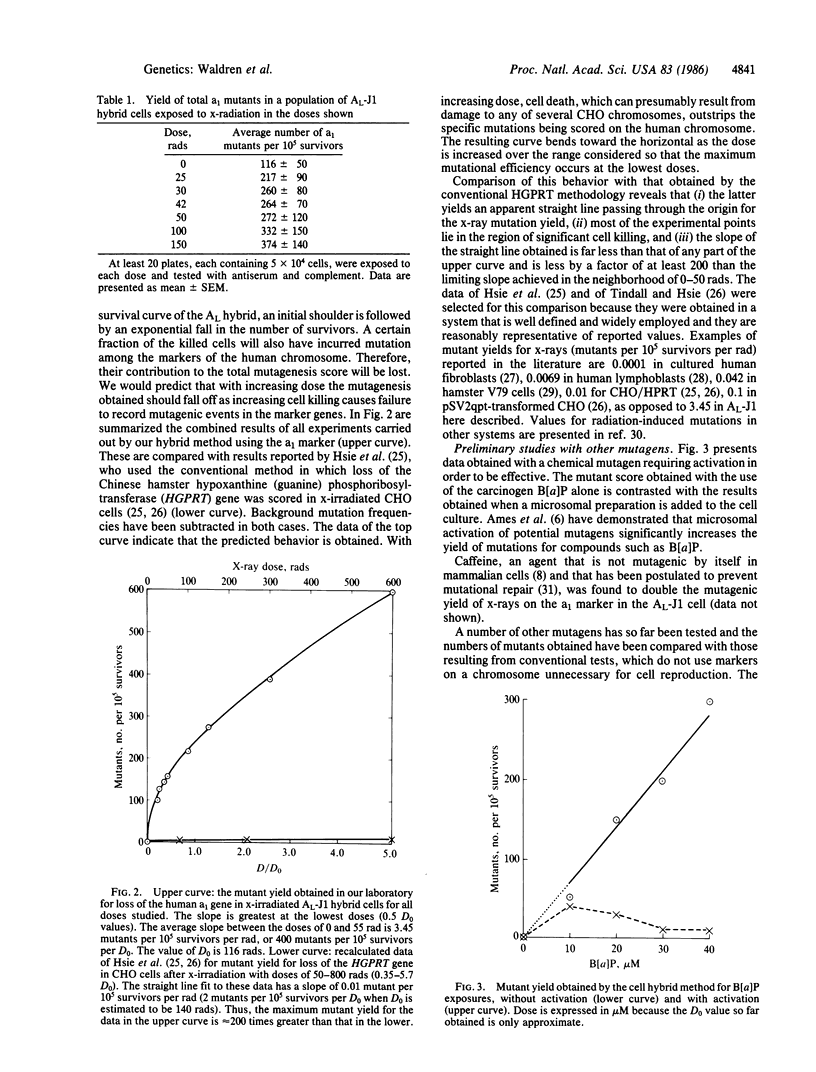
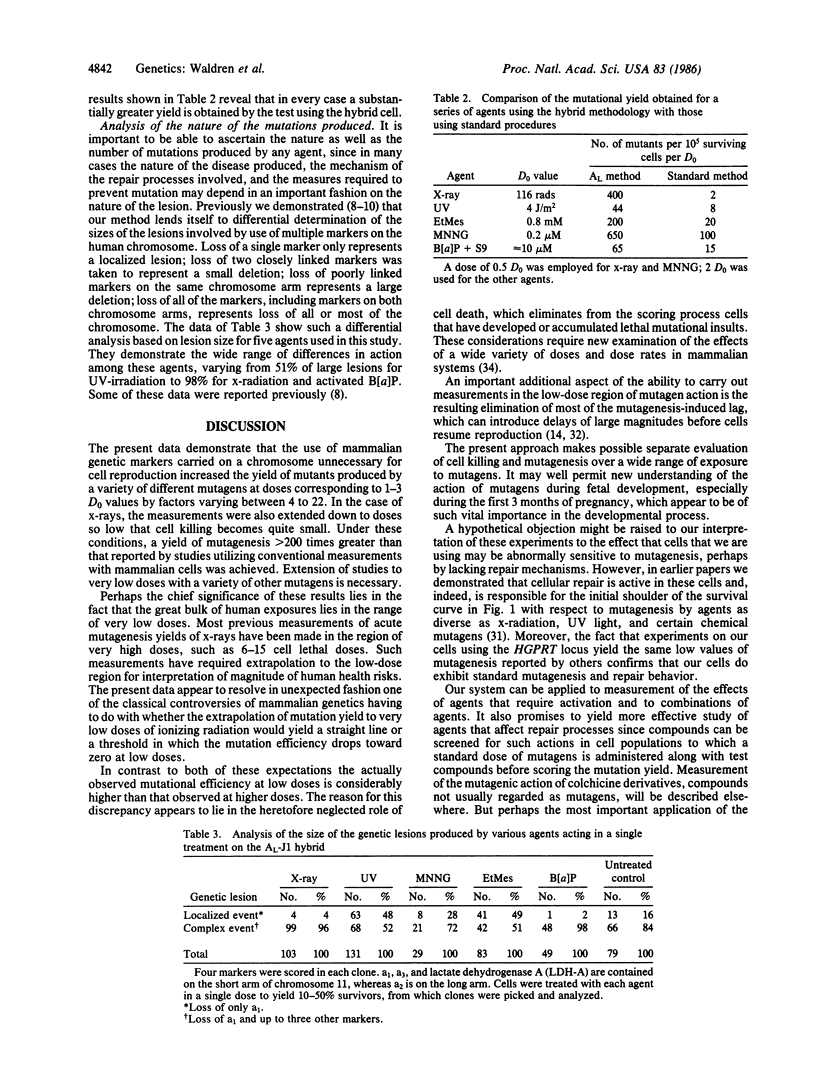
We previously demonstrated that conventional methods for measurement of mutagenesis in mammalian cells are subject to serious error that causes underestimation of environmental contributions to cancer and genetic disease. This error has been corrected by use of somatic cell hybrids containing a single human chromosome on which the marker genes are carried and by using doses of mutagenic agents so low that little cell killing occurs. This method permits direct measurement of the effects of low doses of radiation and other mutagens without resort to the controversial extrapolation procedure customarily used to estimate effects of doses in the neighborhood of actual human exposures. The new data demonstrate that the true mutagenesis efficiency at the low doses of ionizing radiation that approximate human exposures is more than 200 times greater than those obtained with conventional methods. This methodology also permits evaluation of localized mutations, large and small chromosomal deletions, and nondisjunctional processes and can be used for mutagens that need metabolic activation as well as for cooperatively acting agents. The two opposing classical views that in mammalian cells extrapolation to low doses of x-radiation is linear, on the one hand, or involves a threshold, on the other, are both demonstrated to be incorrect at least for the conditions here considered. The actual curve exhibits a downward concavity so that the mutational efficiency is maximal at low doses. These data may have important implications for human health.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., DeMars R. Somatic cell mutation. Detection and quantification of x-ray-induced mutation in cultured, diploid human fibroblasts. Mutat Res. 1973 May;18(2):199–224. doi: 10.1016/0027-5107(73)90037-7. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Chu E. H. Mammalian cell genetics. 3. Characterization of x-ray-induced forward mutations in Chinese hamster cell cultures. Mutat Res. 1971 Jan;11(1):23–34. doi: 10.1016/0027-5107(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Ehling U. H., Averbeck D., Cerutti P. A., Friedman J., Greim H., Kolbye A. C., Jr, Mendelsohn M. L. International Commission for Protection against Environmental Mutagens and Carcinogens. ICPEMC publication no. 10. Review of the evidence for the presence or absence of thresholds in the induction of genetic effects by genotoxic chemicals. Mutat Res. 1983 Dec;123(3):281–341. doi: 10.1016/0165-1110(83)90026-x. [DOI] [PubMed] [Google Scholar]
- Fox M. Factors affecting the quantitation of dose-response curves for mutation induction in V79 Chinese hamster cells after exposure to chemical and physical mutagens. Mutat Res. 1975 Sep;29(3):449–466. doi: 10.1016/0027-5107(75)90064-0. [DOI] [PubMed] [Google Scholar]
- Grosovsky A. J., Little J. B. Evidence for linear response for the induction of mutations in human cells by x-ray exposures below 10 rads. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2092–2095. doi: 10.1073/pnas.82.7.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R. H., Eckardt F. Analysis of dose-response patterns in mutation research. Can J Genet Cytol. 1979 Sep;21(3):277–302. doi: 10.1139/g79-033. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., O'Neill J. P., Couch D. B., SanSebastian J. R., Brimer P. A., Machanoff R., Fuscoe J. C., Riddle J. C., Li A. P., Forbes N. L. Quantitative analyses of radiation- and chemical-induced lethality and mutagenesis in Chinese hamster ovary cells. Radiat Res. 1978 Dec;76(3):471–492. [PubMed] [Google Scholar]
- Jones C., Bill J., Larizza L., Pym B., Goodfellow P., Tunnacliffe A. Relationships between genes on human chromosome 11 encoding cell-surface antigens. Somat Cell Mol Genet. 1984 Jul;10(4):423–428. doi: 10.1007/BF01535638. [DOI] [PubMed] [Google Scholar]
- Jones C., Kimmel K. A., Carey T. E., Miller Y. E., Lehman D. W., MacKenzie D. Further studies on a hybrid cell-surface antigen associated with human chromosome 11 using a monoclonal antibody. Somatic Cell Genet. 1983 Jul;9(4):489–496. doi: 10.1007/BF01543049. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IX. Quantitation of mutagenesis by physical and chemical agents. J Cell Physiol. 1969 Dec;74(3):245–258. doi: 10.1002/jcp.1040740305. [DOI] [PubMed] [Google Scholar]
- Machanoff R., O'Neill J. P., Hsie A. W. Quantitative analysis of cytotoxicity and mutagenicity of benzo[a]pyrene in mammalian cells (CHO/HGPRT system). Chem Biol Interact. 1981 Feb;34(1):1–10. doi: 10.1016/0009-2797(81)90084-3. [DOI] [PubMed] [Google Scholar]
- Muller H. J., Herskowitz I. H., Abrahamson S., Oster I. I. A Nonlinear Relation between X-Ray Dose and Recovered Lethal Mutations in Drosophila. Genetics. 1954 Sep;39(5):741–749. doi: 10.1093/genetics/39.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T. In vitro studies on the radiation biology of mammalian cells. Prog Biophys Mol Biol. 1960;10:237–258. [PubMed] [Google Scholar]
- Thilly W. G. Dead cells don't form mutant colonies: a serious source of bias in mutation assays. Environ Mutagen. 1985;7(2):255–258. doi: 10.1002/em.2860070212. [DOI] [PubMed] [Google Scholar]
- Waldren C. A., Rasko I. Caffeine enhancement of X-ray killing in cultured human and rodent cells. Radiat Res. 1978 Jan;73(1):95–110. [PubMed] [Google Scholar]
- Waldren C., Jones C., Puck T. T. Measurement of mutagenesis in mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1358–1362. doi: 10.1073/pnas.76.3.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldren C., Martin J., Sutherland J., Cram S. Use of somatic cell hybrids for quantitation of mutagenesis: reduction in background mutants by fluorescence-activated cell sorting (FACS). Cytometry. 1984 Nov;5(6):584–588. doi: 10.1002/cyto.990050606. [DOI] [PubMed] [Google Scholar]
- Wuthier P., Jones C., Puck T. T. Surface antigens of mammalian cells as genetic markers. II. J Exp Med. 1973 Jul 1;138(1):229–244. doi: 10.1084/jem.138.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]


