Abstract
Background
The combined associations of changes in cardiorespiratory fitness and body mass index (BMI) with mortality remain controversial and uncertain.
Methods and Results
We examined the independent and combined associations of changes in fitness and BMI with all-cause and cardiovascular disease (CVD) mortality in 14 345 men (mean age 44 years) with at least two medical examinations. Fitness, in metabolic equivalents (METs), was estimated from a maximal treadmill test. BMI was calculated using measured weight and height. Changes in fitness and BMI between the baseline and last examinations over 6.3 years were classified into loss, stable, or gain groups. During 11.4 years of follow-up after the last examination, 914 all-cause and 300 CVD deaths occurred. The hazard ratios (95% confidence intervals) of all-cause and CVD mortality were 0.70 (0.59 to 0.83) and 0.73 (0.54 to 0.98) for stable fitness, and 0.61 (0.51 to 0.73) and 0.58 (0.42 to 0.80) for fitness gain, respectively, compared with fitness loss in multivariable analyses including BMI change. Every 1-MET improvement was associated with 15% and 19% lower risk of all-cause and CVD mortality, respectively. BMI change was not associated with all-cause or CVD mortality after adjusting for possible confounders and fitness change. In the combined analyses, men who lost fitness had higher all-cause and CVD mortality risks regardless of BMI change.
Conclusions
Maintaining or improving fitness is associated with a lower risk of all-cause and CVD mortality in men. Preventing age-associated fitness loss is important for longevity regardless of BMI change.
Keywords: exercise capacity, obesity, mortality, cardiovascular disease, epidemiology
Cardiorespiratory fitness (hereafter fitness) expressed in metabolic equivalents (METs) is a strong independent mortality predictor.1–5 It is a reliable objective marker of habitual physical activity, and a significant diagnostic and prognostic clinical indicator.1,6 Body mass index (BMI) is the most widely accepted measure of overall obesity at the population-level. J-shaped or U-shaped associations between BMI and mortality have been well established.7–9
Previous prospective studies have primarily been limited to a single baseline assessment of either fitness or BMI with subsequent mortality follow-up, and have assumed no changes in fitness and BMI during follow-up. However, the direction and magnitude of changes in fitness and BMI over time vary between individuals and may affect mortality. Prospective studies of fitness change and mortality are sparse,10,11 and the long-term effects of BMI change on mortality have remained controversial,12–14 despite the short-term beneficial effects of weight loss on the metabolic variables associated with obesity.15,16 In addition, changes in lifestyle factors and medical conditions have not been fully considered in many studies. Moreover, although fitness is a strong mortality predictor,17 most studies of BMI and mortality did not take fitness into account.
It is important to study the combined effects of fitness and BMI on mortality as both clinical indicators impact the development of health recommendations and policies. The relative contributions of fitness, physical activity, and obesity to mortality are complicated and continuously debated.17–20 Some report that fitness can eliminate the increased risk of mortality associated with obesity.5,21–23 Others suggest that fitness attenuates, but not eliminates, the adverse effects of obesity on mortality.24,25 If overweight or obese persons, who comprise two-thirds of the U.S. population,26 can reduce the risk of premature mortality by improving physical activity or fitness, this carries a large clinical and public health implication. However, few data are available to access the combined associations of changes in fitness and BMI with mortality in adults.
We examined the independent and combined associations of changes in fitness and BMI with all-cause and cardiovascular disease (CVD) mortality in men using the well-characterized Aerobics Center Longitudinal Study (ACLS). We addressed the questions of whether change in fitness or BMI is associated with mortality, whether their effects are mutually independent of each other, and whether mortality risks differ between the different combinations of changes in fitness and BMI, taking other changes in lifestyle factors and health conditions into account.
Methods
Study Population
The ACLS is a prospective observational study of individuals who received extensive preventive medical evaluations. Study participants were referred by their employers or physicians, or were self-referred. They are primarily non-Hispanic whites (>95%) and college graduates from middle-to-upper socioeconomic strata.27 To assess changes in fitness and BMI, we included men who received at least two medical examinations between 1974 and 2002. For men attending more than two examinations, we used the first (baseline) and last examinations, and followed participants for subsequent mortality after the last examination. Among 16 299 men aged 20 years or older at baseline, we excluded 1381 men reporting myocardial infarction, stroke, or cancer; 34 men with BMI <18.5 kg/m2; and 281 men not reaching 85% of their age-predicted maximal heart rate (220 minus age in years) on an treadmill test at the baseline and/or at the last examination. In addition, 187 men who answered “Yes” to the question about “unexplained weight loss or gain” at the last examination, 53 men with less than 1 year of mortality follow-up, and 13 men with an extreme value of BMI change (>10 kg/m2 change per year) or fitness change (>10 METs change per year) were excluded. For the analyses of CVD mortality, we excluded 614 men who died from causes other than CVD. The final sample included 14 345 men for analyses of all-cause mortality, and 13 731 men for analyses of CVD mortality. The extensive exclusion criteria were used to minimize potential biases due to preexisting diseases or subclinical conditions on changes in fitness and BMI, and its associations with mortality. Because of the small number of women with at least two examinations, this study was limited to men. The study was reviewed and approved annually by the Cooper Institute Institutional Review Board and all participants gave written informed consent for the examinations and follow-up study.
Clinical Examination
Participants completed extensive and comprehensive clinical examinations by a physician. Blood chemistries were analyzed after overnight fast. Resting blood pressure was measured by standard auscultation method after at least 5 minutes of seated rest. Electrocardiogram (ECG) was measured at rest and exercise, and abnormal ECG responses included rhythm and conduction disturbances and ischemic ST-T wave abnormalities. Smoking status, alcohol intake, physical activity, parental CVD, and physician-diagnosed myocardial infarction, stroke, cancer, hypertension, diabetes, and hypercholesterolemia were obtained from the medical questionnaire. Parental CVD was defined as the occurrence of heart attacks, coronary disease, angioplasty, or stroke under age 50 years in either father or mother.
Cardiorespiratory Fitness and Body Mass Index
All participants underwent a symptom-limited maximal treadmill test using the modified Balke protocol.28 In brief, the grade was level at the start, 2% after one minute, and then increased 1% per minute with the speed fixed at 88m/min (3.3 mph). After 25 minutes, the speed was increased 5.4m/min (0.2 mph) without grade change until the participants requested to stop due to exhaustion, or the physician stopped the test for medical reasons. Participants who failed to reach 85% of their age-predicted maximal heart rate were excluded because they were assumed to have subclinical medical conditions, and less than near maximal effort would lead to an underestimate of fitness, which may confound results. Fitness in METs was estimated based on the final treadmill speed and grade using the following formula from the American College of Sports Medicine: [3.5 + (0.1 X speed) + (1.8 X speed X grade)] / 3.5.29 BMI was calculated from measured weight and height (kg/m2), and men were classified into normal weight as BMI 18.5–24.9, overweight as BMI 25.0–29.9, and obese as BMI ≥30.0 kg/m2. Percent body fat in a subgroup of 12 475 men was determined by hydrodensitometry (66%) or skinfold measurement (34%) with standardized procedures. These two measures were highly correlated (r>0.90) for participants who had both measurements.30
Change in fitness and change in BMI as continuous variables were calculated as the difference in maximal METs or BMI between the baseline and last examinations, and divided by the number of years between the two examinations. We used changes in fitness and BMI per year as our main exposures because the intervals between the two examinations varied among individuals in our cohort. We found approximately half of the men increased their maximal METs (47%) and BMI (58%) and the other half showed a decrease or no change. Based on these approximate equal distributions, changes in fitness and BMI per year were categorized into thirds for simplifying the complicated joint associations of changes in fitness and BMI with all-cause and CVD mortality. The lower thirds of changes in maximal METs and BMI showing decreases of fitness and BMI were categorized as “loss”; the middle thirds showing small changes of fitness and BMI were categorized as “stable”; and the upper thirds showing increases of fitness and BMI were categorized as “gain” group (Table 1). For the joint analysis of changes in fitness and BMI with mortality, we created nine combinations from the three fitness and three BMI change categories.
Table 1.
Changes in Fitness and BMI by Thirds of Each Change in 14 345 Men.
| Mean | Median | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Fitness change (maximal METs per year) | |||||
| Loss (n=4782) | −0.33 | −0.22 | 0.35 | −4.61 | −0.06 |
| Stable (n=4781) | 0.04 | 0 | 0.07 | −0.06 | 0.19 |
| Gain (n=4782) | 0.80 | 0.52 | 0.78 | 0.19 | 9.95 |
| BMI change (kg/m2 per year) | |||||
| Loss (n=4781) | −0.65 | −0.34 | 0.93 | −9.79 | −0.06 |
| Stable (n=4782) | 0.05 | 0.05 | 0.06 | −0.06 | 0.15 |
| Gain (n=4782) | 0.46 | 0.33 | 0.42 | 0.15 | 7.98 |
In addition, we investigated the associations of changes in fitness and BMI status as categorical variables with mortality to further consider the baseline levels of fitness and BMI. We dichotomized fitness into either unfit (lower 20%) or fit (upper 80%) based on their age-specific (20–39, 40–49, 50–59, and ≥60 years) fifths of treadmill time distributions from the entire ACLS cohort, following our previous ACLS studies,5,10,22,23 which have shown unfit to be a strong independent morbidity and mortality predictor, given the fact that there has been no consensus for the clinical definition of fitness. We combined overweight and obese men due to the small number of obese men. Four different patterns of category changes in fitness and BMI were defined as followed: fit or normal weight at both the baseline and last examinations; fit or normal weight at the baseline but unfit or overweight or obese at the last examination; unfit or overweight or obese at the baseline but fit or normal weight at the last examination; and unfit or overweight or obese at both examinations.
To examine a dose-response relationship of changes in maximal METs, body weight, or % body fat with mortality, changes in each continuous variable were further classified into fifths of each change per year of interval between the baseline and last examinations.
Mortality Surveillance
We followed participants for mortality from the last examination through the date of death for decedents or December 31, 2003 for survivors using the National Death Index. We excluded men with less than one year of follow-up to minimize potential bias due to serious underlying illness on mortality. Also, we excluded early deaths during the first three years of follow-up in an additional analysis.
Statistical Analysis
Baseline differences between survivors and decedents were assessed using χ2 for categorical variables and t test for continuous variables. We used Cox proportional hazard models to estimate the hazard ratios and 95% confidence intervals for all-cause and CVD mortality across changes in fitness and BMI. Analyses were adjusted for age, year of examination, parental CVD, BMI, and maximal METs at baseline, number of clinic visits between the baseline and last examinations, dummy variables for the combination patterns of each lifestyle factor (smoking status, alcohol intake, and physical activity) and the combination patterns of each medical condition (abnormal electrocardiogram, hypertension, diabetes, and hypercholesterolemia) at the baseline and last examinations, and changes in BMI or maximal METs for each other. We defined four combination patterns of each lifestyle factor (remained non-smokers, became non-smokers, became smokers, or remained smokers; remained non-heavy drinkers, became non-heavy drinkers, became heavy drinkers, or remained heavy drinkers; and remained active, became active, became inactive, or remained inactive) and three combination patterns of each medical condition (e.g., remained normotensive, became hypertensive, or remained hypertensive) at the two examinations. The proportional hazards assumption was satisfied when comparing the log-log survival plots grouped on exposure categories. There were no significant interactions between fitness change and BMI change on all-cause and CVD mortality risks using interaction terms in the Cox regression. We used 2-sided P values less than 0.05 to indicate statistical significance using SAS software (version 9.2).
Results
The mean (interquartile range) interval between the baseline and last examinations was 6.3 (7.0) years with a range of 2 months to 28.7 years, and there were 914 deaths from all-causes and 300 deaths from CVD during 11.4 (14.8) years of follow-up among 14 345 adult men. At baseline (Table 2), survivors were younger, fitter, more active, and less likely to be smokers compared with decedents. Also, survivors were more likely to show favorable profiles in the medical conditions. In general, the current study population comprised middle-aged, slightly overweight (mean BMI 26.0 and range 18.6 to 57.3 kg/m2), and relatively fit men (mean maximal MET 11.9 and range 4.4 to 25.8) at baseline.
Table 2.
Baseline Characteristics by Survival Status in 14 345 Men.
| All (n=14 345) |
Survivors (n=13 431) |
Decedents (n=914) |
P | |
|---|---|---|---|---|
| Age, y | 43.7 (9.1) | 43.2 (8.8) | 50.7 (9.9) | <0.001 |
| Body weight, kg | 83.7 (12.1) | 83.8 (12.1) | 82.8 (12.1) | 0.03 |
| Body mass index, kg/m2 | 26.0 (3.3) | 26.0 (3.3) | 26.0 (3.2) | 0.92 |
| 18.5–24.9, % | 41.8 | 41.9 | 41.2 | |
| 25.0–29.9, % | 47.4 | 47.3 | 48.0 | 0.91 |
| ≥30, % | 10.8 | 10.8 | 10.8 | |
| Cardiorespiratory fitness, maximal METs | 11.9 (2.3) | 12.0 (2.3) | 10.5 (2.2) | <0.001 |
| Systolic blood pressure, mm Hg | 121 (13) | 121 (13) | 126 (15) | <0.001 |
| Diastolic blood pressure, mm Hg | 81 (9) | 81 (9) | 83 (10) | <0.001 |
| Hypertension, %* | 29 | 28 | 45 | <0.001 |
| Fasting glucose, mg/dL | 100.0 (15.2) | 99.7 (14.7) | 103.7 (20.6) | <0.001 |
| Diabetes, %† | 5 | 5 | 13 | <0.001 |
| Total cholesterol, mg/dL | 208.6 (39.0) | 208.1 (39.0) | 216.1 (37.9) | <0.001 |
| Hypercholesterolemia, %‡ | 26 | 26 | 29 | 0.04 |
| Abnormal electrocardiogram, %§ | 6 | 6 | 14 | <0.001 |
| Current smoker, % | 16 | 16 | 23 | <0.001 |
| Heavy drinker, %‖ | 18 | 18 | 19 | 0.31 |
| Physically inactive, %# | 28 | 27 | 38 | <0.001 |
| Parental CVD, % | 28 | 27 | 39 | <0.001 |
Data are presented as mean (SD) unless otherwise indicated.
Defined as systolic or diastolic blood pressure ≥140/90mmHg or physician diagnosis.
Defined as fasting glucose ≥126 mg/dl, current therapy with insulin, or physician diagnosis.
Defined as total cholesterol ≥240 mg/dl or physician diagnosis.
Defined as abnormal resting or exercise electrocardiogram.
Defined as >14 alcohol drinks per week.
Defined as no leisure-time physical activity in the past 3 months before the examination.
Compared with men who lost fitness (Table 3), men who maintained fitness had 30% and 28% lower risk of all-cause and CVD mortality, respectively, men who improved fitness had 40% and 44% lower risk of corresponding mortality after adjusting for possible baseline confounders and changes in lifestyle factors and medical conditions (Model 1). These associations remained nearly the same after further adjusting for BMI change (Model 2). Each 1-MET improvement in fitness was associated with 15% and 19% lower risk of all-cause and CVD mortality, respectively, after adjusting for possible confounders and BMI change (Model 2). However, BMI change was not significantly associated with all-cause mortality, but men who gained BMI had a higher risk of CVD mortality compared with men who lost BMI after adjusting for possible confounders (Model 1). When we further adjusted for maximal MET change (Model 2), the magnitude of associations between BMI change and all-cause and CVD mortality were further attenuated, and the higher risk of CVD mortality in men who gained BMI was no longer significant. Each 1-unit increase in BMI was associated with 6% and 8% higher risk of all-cause and CVD mortality, respectively (Model 1). However, these associations were also no longer significant after further adjusting for maximal MET change (Model 2).
Table 3.
Hazard Ratios of All-Cause and Cardiovascular Disease Mortality by Changes in Fitness and BMI in 14 345 Men.
| All-Cause Mortality | CVD Mortality | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||
| Model 1* | Model 2† | Model 1* | Model 2† | |
| Fitness change | ||||
| Loss | 1.00 | 1.00 | 1.00 | 1.00 |
| Stable | 0.70 (0.59–0.82) | 0.70 (0.59–0.83) | 0.72 (0.54–0.97) | 0.73 (0.54–0.98) |
| Gain | 0.60 (0.50–0.71) | 0.61 (0.51–0.73) | 0.56 (0.41–0.76) | 0.58 (0.42–0.80) |
| P for linear trend | <0.001 | <0.001 | <0.001 | 0.001 |
| Per 1-MET increase | 0.84 (0.80–0.88) | 0.85 (0.80–0.89) | 0.80 (0.74–0.88) | 0.81 (0.74–0.89) |
| BMI change | ||||
| Loss | 1.00 | 1.00 | 1.00 | 1.00 |
| Stable | 1.14 (0.96–1.35) | 1.06 (0.90–1.26) | 1.04 (0.76–1.42) | 0.98 (0.71–1.34) |
| Gain | 1.15 (0.98–1.36) | 1.03 (0.87–1.23) | 1.39 (1.05–1.84) | 1.26 (0.94–1.69) |
| P for linear trend | 0.08 | 0.68 | 0.03 | 0.14 |
| Per 1-BMI increase | 1.06 (1.01–1.10) | 1.03 (0.98–1.08) | 1.08 (1.01–1.17) | 1.06 (0.97–1.14) |
Adjusted for age, examination year, parental CVD, BMI, and maximal METs at baseline, the combination patterns of each lifestyle factor (smoking status, alcohol intake, and physical activity) and each medical condition (abnormal electrocardiogram, hypertension, diabetes, and hypercholesterolemia) at the baseline and last examinations, and the number of clinic visits between the baseline and last examinations.
Adjusted for model 1 plus BMI change (for fitness change) or maximal MET change (for BMI change) between the baseline and last examinations.
For fitness and BMI status change (Table 4), men who became fit or remained fit had approximately 40–50% lower risks of all-cause and CVD mortality compared with men who remained unfit, whereas BMI status change was not significantly associated with either all-cause or CVD mortality, after adjusting for possible confounders and BMI change or fitness change for each other. Men who became unfit had a higher risk of CVD mortality compared with men who remained unfit even after adjusting for BMI change.
Table 4.
Hazard Ratios of All-Cause and Cardiovascular Disease Mortality by Changes in Fitness Status and BMI Status in 14 345 Men.
| All-Cause Mortality | CVD Mortality | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||
| Model 1* | Model 2† | Model 1* | Model 2† | |
| Fitness status change‡ | ||||
| Remained unfit | 1.00 | 1.00 | 1.00 | 1.00 |
| Became unfit | 1.40 (0.97–2.02) | 1.38 (0.95–1.99) | 1.95 (1.07–3.56) | 1.89 (1.03–3.45) |
| Became fit | 0.52 (0.39–0.69) | 0.53 (0.40–0.71) | 0.58 (0.36–0.92) | 0.59 (0.37–0.95) |
| Remained fit | 0.52 (0.40–0.66) | 0.52 (0.40–0.66) | 0.56 (0.36–0.85) | 0.56 (0.37–0.85) |
| BMI status change | ||||
| Remained normal weight | 1.00 | 1.00 | 1.00 | 1.00 |
| Became normal weight | 1.00 (0.77–1.29) | 1.06 (0.82–1.38) | 1.15 (0.74–1.78) | 1.24 (0.80–1.93) |
| Became overweight or obese | 1.11 (0.85–1.44) | 1.05 (0.80–1.36) | 1.00 (0.59–1.70) | 0.95 (0.56–1.61) |
| Remained overweight or obese | 1.04 (0.89–1.22) | 1.03 (0.88–1.20) | 1.20 (0.91–1.59) | 1.19 (0.90–1.57) |
Adjusted for age, examination year, parental CVD, and BMI (for fitness change) or maximal METs (for BMI change) at baseline, the combination patterns of each lifestyle factor (smoking status, alcohol intake, and physical activity) and each medical condition (abnormal electrocardiogram, hypertension, diabetes, and hypercholesterolemia) at the baseline and last examinations, and the number of clinic visits between the baseline and last examinations.
Adjusted for model 1 plus BMI change (for fitness change) or maximal MET change (for BMI change) between the baseline and last examinations.
Unfit was defined as the least fit 20% and fit was defined as most fit 80% of maximal treadmill time.
In additional analyses stratified by baseline fitness status or BMI status, fitness change was inversely associated with all-cause and CVD mortality among both unfit and fit men at the baseline (all P for linear trend <0.05), however no significant associations were found between BMI change and either mortality among both normal weight and overweight or obese men (data not shown). To minimize potential biases caused by subclinical conditions on mortality, we additionally excluded early deaths occurred in the first three years of follow-up. The results were virtually the same showing inverse associations between fitness change and all-cause and CVD mortality (both P for linear trend <0.001) and no associations between BMI change and either mortality (data not shown).
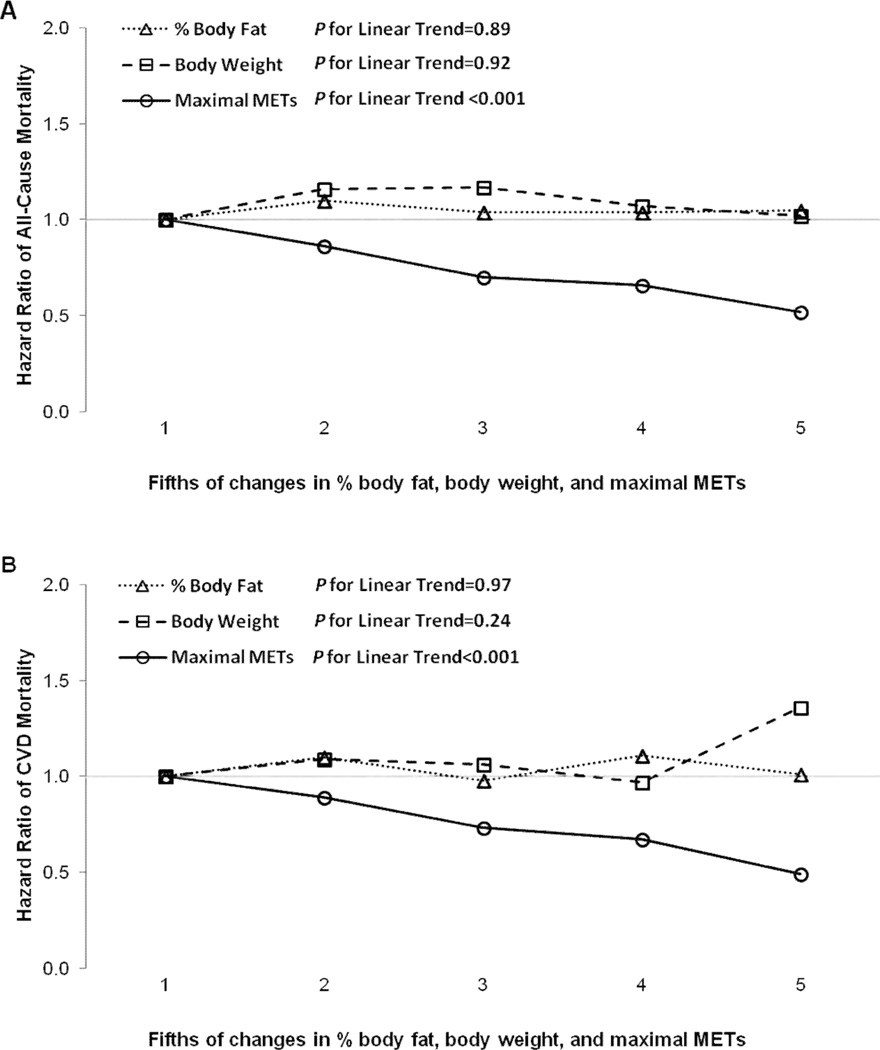
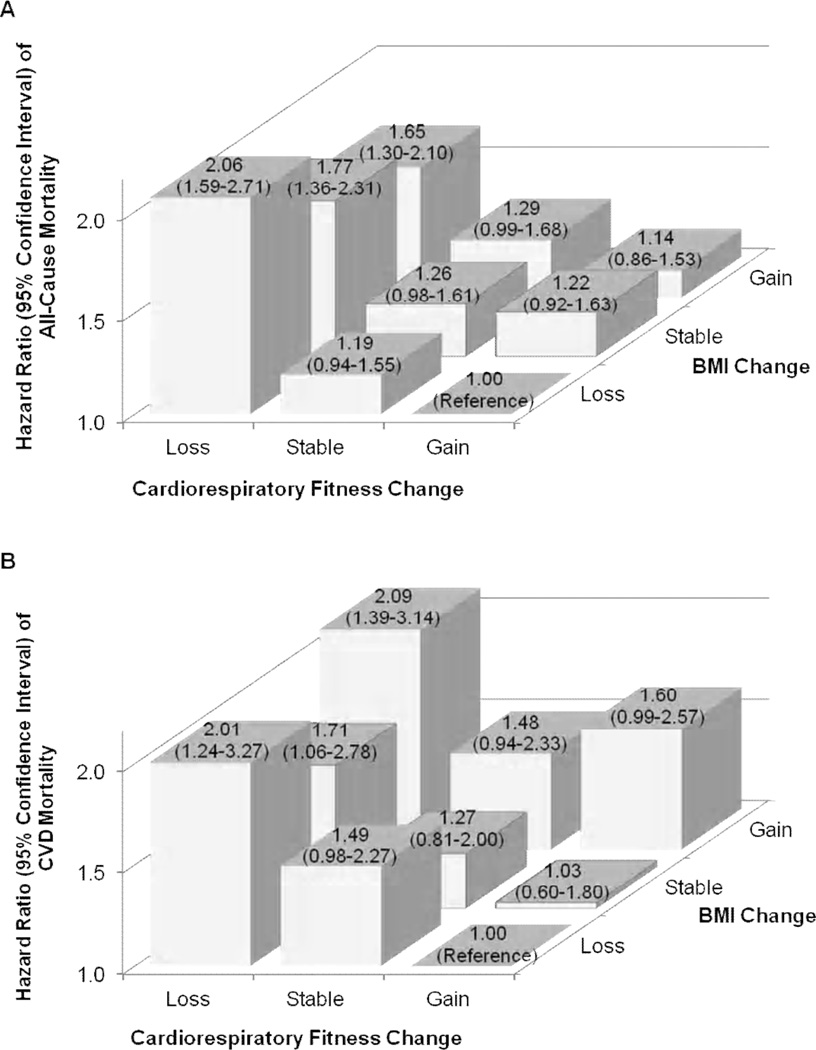
Significant inverse dose-response relationships were observed between fifths of change in maximal METs and all-cause and CVD mortality (both P for linear trend <0.001), however, there were no significant trends in either mortality across the fifths of changes in % body fat and body weight (Figure 1). In the combined analyses of changes in fitness and BMI (Figure 2), men who lost fitness had a higher risk of both all-cause and CVD mortality regardless of BMI change, compared with the reference group, men who improved fitness and lost BMI. An increase in BMI was not significantly associated with all-cause or CVD mortality when combined with maintaining or improving fitness. We provided characteristics at baseline and last examinations by nine combinations of changes in fitness and BMI in the online supplemental table.
Figure 1.
Hazard ratios of all-cause (A) and cardiovascular disease (B) mortality by fifths of changes in % body fat (data from 12 475 men), body weight, and maximal METs in 14 345 men. All data were adjusted for age, examination year, parental CVD, baseline value of each exposure, the combination patterns of each lifestyle factor (smoking status, alcohol intake, and physical activity) and each medical condition (abnormal electrocardiogram, hypertension, diabetes, and hypercholesterolemia) at the baseline and last examinations, the number of clinic visits between the baseline and last examinations, and baseline maximal METs and maximal MET change (for % body fat change and body weight change) or baseline weight and weight change (for maximal MET change).
Figure 2.
Hazard ratios (95% confidence intervals) of all-cause (A) and cardiovascular disease (B) mortality by combinations of changes in fitness and BMI in 14 345 men. All data were adjusted for age, examination year, parental CVD, BMI, and maximal METs at baseline, the combination patterns of each lifestyle factor (smoking status, alcohol intake, and physical activity) and each medical condition (abnormal electrocardiogram, hypertension, diabetes, and hypercholesterolemia) at the baseline and last examinations, and the number of clinic visits between the baseline and last examinations. The number of men (number of all-cause deaths) in the fitness loss, stable, and gain groups were 717 (82), 1240 (91), and 2824 (208) in the BMI loss group; 1732 (101), 2129 (113), and 921 (63) in the stable BMI group; and 2333 (115), 1412 (79), and 1037 (62) in the BMI gain group, respectively. Also, the number of men (number of CVD deaths) in the fitness loss, stable, and gain groups were 658 (23), 1184 (35), and 2686 (70) in the BMI loss group; 1660 (29), 2050 (34), and 874 (16) in the stable BMI group; and 2259 (41), 1361 (28), and 999 (24) in the BMI gain group, respectively.
Discussion
The primary finding of this study is that maintaining or improving fitness was associated with a lower risk of both all-cause and CVD mortality compared with losing fitness in 14 345 adult men, during 11.4 years of follow-up. Every 1 maximal MET improvement was associated with 15% and 19% lower risk of all-cause and CVD mortality, respectively. Also, men who became fit or remained fit had a lower risk of all-cause and CVD mortality compared with men who remained unfit. These associations were observed after accounting for possible confounding effects of baseline risk factors, changes in lifestyle factors and medical conditions, and simultaneous change in BMI. Moreover, these findings were consistent regardless of their baseline fitness levels, and exclusion of early deaths did not alter the results. Only two previous cohort studies on fitness change and mortality reported similar results, indicating a reduced mortality risk with improvements in fitness. Our earlier ACLS report found that both healthy and unhealthy men who maintained or improved fitness were less likely to die over 5 years of follow-up.10 A Norwegian study found an inverse relation between fitness change and mortality in 1428 men aged 40–60 at baseline after excluding those with any recognized disease.11 Compared with these previous studies, our current study has much larger sample size over a wider age range (20 to 100 years at baseline) and a longer follow-up time. We also take changes in lifestyle factors, medical conditions, and BMI into account. A recent meta-analysis on the association between a single assessment of fitness and mortality reported that a 1-MET higher level of fitness at baseline was associated with 13% lower risk of all-cause mortality during follow-up.2 Our current results of 15% and 19% lower risk of all-cause and CVD mortality, respectively, with every 1-MET improvement after further adjusting for BMI change confirms the strong effects of not only baseline fitness but also fitness change on all-cause and CVD mortality, independently of simultaneous change in BMI.
The most important original finding from our combined analysis is that men who lost fitness had a higher mortality risk regardless of BMI change compared with the reference group, men who improved fitness and lost BMI. However, men who maintained or improved their fitness were more likely to attenuate the potentially negative effects of BMI increase on all-cause and CVD mortality. Because we could not find similar studies examining the combined associations of changes in fitness and BMI with mortality, we could not directly compare our results with others. However, in our earlier studies on the joint associations of fitness and BMI at a single baseline assessment with mortality, we found comparable results indicating the higher risk of all-cause and CVD mortality associated with being unfit regardless of BMI status in adult men,22 older adults,5 and men with diabetes21 or hypertension.23 Also, other studies reported a higher risk of all-cause and CVD mortality in unfit men regardless of their BMI status.24,25
In the relations between change in fitness and changes in lifestyle factors and medical conditions, men who became sedentary, started smoking, or developed disease such as hypertension or diabetes were more likely to decrease their fitness levels, after adjusting for BMI change (data not shown), as stated earlier.3,10,31,32 The strongest association was observed between changes in physical activity and fitness indicating that among men who became active, 80% of them maintained or improved their fitness, whereas among men who became sedentary, 47% of them lost their fitness levels (data not shown). Although fitness has some genetic components, it is suggested that physical activity is likely one of the important mechanisms explaining fitness change.
In this study, BMI change was not significantly associated with all-cause mortality, and the observed higher risk of CVD mortality associated with BMI gain was no longer significant once fitness change was taken into account, indicating modifiable effects by fitness change on the association between BMI change and CVD mortality. These findings were consistent after further consideration of subclinical conditions by excluding early deaths, and also irrespective of baseline BMI status. Although overweight and obesity defined at a single baseline assessment are well-established mortality predictors,7–9,33 prospective studies of long-term BMI change and mortality have remained controversial. Some studies showed similar results to ours, indicating no significant associations between BMI or weight change and all-cause or CVD mortality after taking preexisting diseases into account.34–36 Others reported that BMI or weight loss is associated with a higher mortality risk.37–40 However, it is suggested that the association between BMI or weight loss and a higher mortality risk may be due to failure to control medical conditions.34,36,41 In fact, many studies reporting a higher risk of mortality associated with BMI or weight loss appeared to have some limitations due to lack of adequate health and medical information or failure to control for preexisting diseases or subclinical conditions, leading to both weight loss and a high risk of mortality. We excluded men with CVD, cancer, underweight (BMI<18.5 kg/m2), or men not reaching 85% of age-predicted maximal heart rate on the treadmill test not only at the baseline but also at the last examination prior to the subsequent mortality follow-up. We also excluded men reporting unexplained weight change at the last examination. Furthermore, changes in lifestyle factors and medical conditions were taken into account in the analysis. We believe these extensive exclusion criteria and comprehensive analysis can minimize the possible bias from preexisting or subclinical conditions on the associations of changes in fitness and BMI with mortality. Because none of the previous studies on weight change have considered fitness change in their analyses, it is hard to assess whether BMI or weight change is associated with mortality independent of fitness change in other studies.
There are several other important issues to consider regarding BMI or weight loss and mortality. First, some observational studies show that intentional weight loss may be beneficial to longevity,13,42,43 but recent reviews report conflicting findings in studies on intentional weight loss and mortality requiring well-designed further studies.13,15 Although overall weight loss may be deleterious, fat loss may possibly be associated with greater longevity, as suggested from the Tecumseh Community Health Study and the Framingham Heart Study.37 However, our data showed no significant trends in all-cause or CVD mortality across fifths of change in % body fat, similar to BMI change. There is evidence that weight variability or fluctuation is associated with higher mortality risk.34,35,44,45 An additional analysis in our sample with 8150 men, with at least three medical examinations, indicated that every 1 kg of weight variability (defined as intrapersonal standard deviation of weight change) was associated with higher mortality risk even after adjusting for BMI change (data not shown). Therefore, it is still possible that weight variability instead of weight loss or gain may be associated with a higher mortality risk. The Finnish Twin Cohort study observed no excess mortality risk following weight loss among those exercising, but found a higher mortality risk in those dieting to lose weight.46 It is therefore important to determine how different methods used for weight loss can affect mortality, and well-designed randomized clinical trials are needed.
This study expands our knowledge and understanding of the roles of fitness and obesity on mortality by exploring the independent and combined associations of changes in fitness and BMI with all-cause and CVD mortality. A relatively large sample size across a wide age-range, extensive mortality follow-up, objectively-measured fitness and BMI, and extensive control of potential biases caused by preexisting diseases or subclinical conditions strengthen our findings. A major limitation is that our sample consists of well-educated white men from middle-to-upper socioeconomic strata. However, physiologic characteristics of men in the ACLS are similar to representative population samples.27 Also, the socioeconomic homogeneity reduces the possible confounding effects of education, income, and ethnicity. The current results may not apply to severely obese or extremely unfit individuals because the population in this study was, on average, slightly overweight with 90% having a BMI of 30 kg/m2 or under, and relatively fit at baseline. It is possible that change in BMI may be more important on mortality in individuals who are morbidly obese. We could not take into account dietary factors due to lack of adequate dietary information. However, in a subgroup of 11 795 men who reported the number of meals per week, additional adjustment for dietary changes did not alter our findings.
In conclusion, maintaining or improving fitness is associated with a lower risk of premature deaths from all-causes and CVD in adult men. Preventing fitness loss with age, regardless of whether BMI changes, is important for mortality risk reduction. Also, maintaining or improving fitness may attenuate some potentially negative effects of weight gain on mortality. To date, extensive attention has been given to weight loss. However, the long-term effect of fitness change, primarily resulting from increasing physical activity, is likely to be at least as important as weight loss, if not more important, for reducing premature mortality. Increased attention needs to be placed on strategies to maintain or improve fitness.
Supplementary Material
Acknowledgment
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
Sources of Funding
This study was supported by the National Institutes of Health grants (AG06945, HL62508, and DK088195), and an unrestricted research grant from The Coca-Cola Company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Duck-chul Lee, Department of Exercise Science, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA.
Xuemei Sui, Department of Exercise Science, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA.
Enrique G. Artero, Department of Exercise Science, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA, and Department of Medical Physiology, School of Medicine, University of Granada, Granada, Spain.
I-Min Lee, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, and Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
Timothy S. Church, Department of Preventive Medicine Research, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Paul A. McAuley, Department of Human Performance and Sport Sciences, Winston-Salem State University, Winston-Salem, NC, USA.
Fatima C. Stanford, Departments of Internal Medicine and Pediatrics, University of South Carolina School of Medicine, and Palmetto Health Richland Hospital, Columbia, SC, USA.
Harold W. Kohl, III, Division of Epidemiology and Disease Control, University of Texas Health Science Center – Houston, School of Public Health, Michael & Susan Dell Center for Advancement of Healthy Living and Department of Kinesiology and Health Education, University of Texas at Austin, Austin, TX, USA.
Steven N. Blair, Departments of Exercise Science and Epidemiology/Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA.
References
- 1.Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN, Lauer MS, Marwick TH, Pandey DK, Wicklund RH, Thisted RA. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 2.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 3.Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24:27–35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 5.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD, Winters WL, Jr, Yanowitz FG, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Lewis RP, O'Rourke RA, Ryan TJ. ACC/AHA guidelines for exercise testing: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) Circulation. 1997;96:345–354. doi: 10.1161/01.cir.96.1.345. [DOI] [PubMed] [Google Scholar]
- 7.Berrington de GA, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, nton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 9.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, Samet JM. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 10.Blair SN, Kohl HW, III, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 11.Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 12.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009;22:93–108. doi: 10.1017/S0954422409990035. [DOI] [PubMed] [Google Scholar]
- 13.Poobalan AS, Aucott LS, Smith WC, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev. 2007;8:503–513. doi: 10.1111/j.1467-789X.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen MK, Hundrup YA, Obel EB, Gronbaek M, Heitmann BL. Intentional weight loss and mortality among initially healthy men and women. Nutr Rev. 2008;66:375–386. doi: 10.1111/j.1753-4887.2008.00047.x. [DOI] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 17.Lee DC, Sui X, Blair SN. Does physical activity ameliorate the health hazards of obesity? Br J Sports Med. 2009;43:49–51. doi: 10.1136/bjsm.2008.054536. [DOI] [PubMed] [Google Scholar]
- 18.Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev. 2010;11:202–221. doi: 10.1111/j.1467-789X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 19.Hainer V, Toplak H, Stich V. Fat or fit: what is more important? Diabetes Care. 2009;32 Suppl 2:S392–S397. doi: 10.2337/dc09-S346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 21.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 22.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 23.McAuley PA, Sui X, Church TS, Hardin JW, Myers JN, Blair SN. The joint effects of cardiorespiratory fitness and adiposity on mortality risk in men with hypertension. Am J Hypertens. 2009;22:1062–1069. doi: 10.1038/ajh.2009.122. [DOI] [PubMed] [Google Scholar]
- 24.Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156:832–841. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 25.Stevens J, Evenson KR, Thomas O, Cai J, Thomas R. Associations of fitness and fatness with mortality in Russian and American men in the lipids research clinics study. Int J Obes Relat Metab Disord. 2004;28:1463–1470. doi: 10.1038/sj.ijo.0802770. [DOI] [PubMed] [Google Scholar]
- 26.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 27.Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness. Evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol. 1989;129:1145–1156. doi: 10.1093/oxfordjournals.aje.a115236. [DOI] [PubMed] [Google Scholar]
- 28.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 29.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 30.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 31.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. [Accessed March 3. 2011];2008 October; Available at: http://health.gov/PAGuidelines.
- 32.Jackson AS, Sui X, Hebert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781–1787. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 34.Iribarren C, Sharp DS, Burchfiel CM, Petrovitch H. Association of weight loss and weight fluctuation with mortality among Japanese American men. N Engl J Med. 1995;333:686–692. doi: 10.1056/NEJM199509143331102. [DOI] [PubMed] [Google Scholar]
- 35.Rzehak P, Meisinger C, Woelke G, Brasche S, Strube G, Heinrich J. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol. 2007;22:665–673. doi: 10.1007/s10654-007-9167-5. [DOI] [PubMed] [Google Scholar]
- 36.Wannamethee SG, Shaper AG, Walker M. Weight change, body weight and mortality: the impact of smoking and ill health. Int J Epidemiol. 2001;30:777–786. doi: 10.1093/ije/30.4.777. [DOI] [PubMed] [Google Scholar]
- 37.Allison DB, Zannolli R, Faith MS, Heo M, Pietrobelli A, VanItallie TB, Pi-Sunyer FX, Heymsfield SB. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord. 1999;23:603–611. doi: 10.1038/sj.ijo.0800875. [DOI] [PubMed] [Google Scholar]
- 38.Droyvold WB, Lund Nilsen TI, Lydersen S, Midthjell K, Nilsson PM, Nilsson JA, Holmen J, Nord-Trondelag HS. Weight change and mortality: the Nord-Trondelag Health Study. J Intern Med. 2005;257:338–345. doi: 10.1111/j.1365-2796.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 39.Sauvaget C, Ramadas K, Thomas G, Vinoda J, Thara S, Sankaranarayanan R. Body mass index, weight change and mortality risk in a prospective study in India. Int J Epidemiol. 2008;37:990–1004. doi: 10.1093/ije/dyn059. [DOI] [PubMed] [Google Scholar]
- 40.Strandberg TE, Strandberg AY, Salomaa VV, Pitkala KH, Tilvis RS, Sirola J, Miettinen TA. Explaining the obesity paradox: cardiovascular risk, weight change, and mortality during long-term follow-up in men. Eur Heart J. 2009;30:1720–1727. doi: 10.1093/eurheartj/ehp162. [DOI] [PubMed] [Google Scholar]
- 41.Lee IM, Paffenbarger RS., Jr Is weight loss hazardous? Nutr Rev. 1996;54:S116, S124. doi: 10.1111/j.1753-4887.1996.tb03906.x. [DOI] [PubMed] [Google Scholar]
- 42.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med. 2003;138:383–389. doi: 10.7326/0003-4819-138-5-200303040-00007. [DOI] [PubMed] [Google Scholar]
- 43.Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005;165:1035–1040. doi: 10.1001/archinte.165.9.1035. [DOI] [PubMed] [Google Scholar]
- 44.Blair SN, Shaten J, Brownell K, Collins G, Lissner L. Body weight change, all-cause mortality, and cause-specific mortality in the Multiple Risk Factor Intervention Trial. Ann Intern Med. 1993;119:749–757. doi: 10.7326/0003-4819-119-7_part_2-199310011-00024. [DOI] [PubMed] [Google Scholar]
- 45.Diaz VA, Mainous AG, III, Everett CJ. The association between weight fluctuation and mortality: results from a population-based cohort study. J Community Health. 2005;30:153–165. doi: 10.1007/s10900-004-1955-1. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen TI, Rissanen A, Korkeila M, Kaprio J. Intention to lose weight, weight changes, and 18-y mortality in overweight individuals without co-morbidities. PLoS Med. 2005;2:e171. doi: 10.1371/journal.pmed.0020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.