Abstract
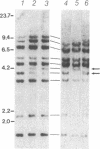
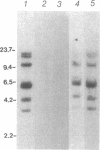
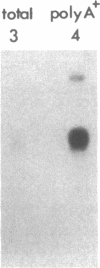
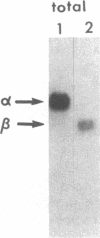
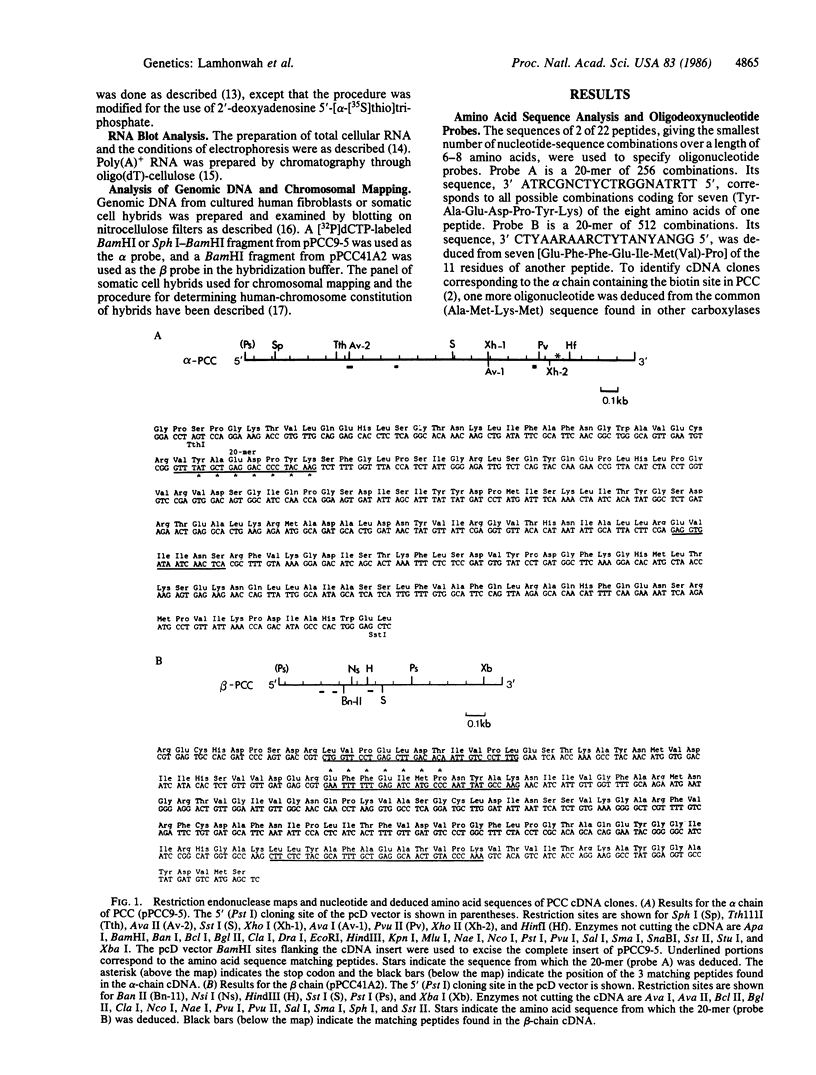
Propionyl-CoA carboxylase [PCC, propanoyl-CoA:carbon-dioxide ligase (ADP-forming), EC 6.4.1.3] is a biotin-dependent enzyme involved in the degradation of branched-chain amino acids, fatty acids with odd-numbered chain lengths, and other metabolites. Inherited deficiency of the enzyme results in propionic acidemia, an autosomal recessive disorder showing considerable clinical heterogeneity. To facilitate investigations of enzyme structure and the nature of mutation in propionic acidemia, we have isolated cDNA clones coding for the alpha and beta polypeptides of human PCC. Sequences of two peptides derived from human liver PCC were used to specify oligonucleotide probes that were then used to screen a human fibroblast cDNA library. Two classes of cDNA clones were thus identified. One class contained the anticipated Ala-Met-Lys-Met sequence, corresponding to the biotin binding site found in several biotin-dependent carboxylases, thus confirming the alpha-chain assignment of these clones. In addition, they contained the deduced amino acid sequence of two of the sequenced peptides, including that of one of the oligonucleotide probes. The second class, coding for the beta polypeptide, contained the sequences of four peptides, including the sequence corresponding to the other oligonucleotide probe. Blot hybridization of RNA from normal human fibroblasts revealed a single mRNA species of 2.9 kilobases coding for the alpha polypeptide and two species of 4.5 and 2.0 kilobases detected for the beta polypeptide. By use of a panel of somatic mouse-human hybrids, the human gene encoding the alpha polypeptide (PCCA) was localized to chromosome 13, while the gene encoding the beta polypeptide (PCCB) was assigned to chromosome 3. Restriction fragment length polymorphisms were identified, at both PCCA and PCCB, that should prove useful to individual families at risk for propionic acidemia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt I. K., Hsia Y. E., Clement D. H., Provence S. A. Propionicacidemia (ketotic hyperglycinemia): dietary treatment resulting in normal growth and development. Pediatrics. 1974 Mar;53(3):391–395. [PubMed] [Google Scholar]
- Freytag S. O., Collier K. J. Molecular cloning of a cDNA for human pyruvate carboxylase. Structural relationship to other biotin-containing carboxylases and regulation of mRNA content in differentiating preadipocytes. J Biol Chem. 1984 Oct 25;259(20):12831–12837. [PubMed] [Google Scholar]
- Frischauf A. M., Garoff H., Lehrach H. A subcloning strategy for DNA sequence analysis. Nucleic Acids Res. 1980 Dec 11;8(23):5541–5549. doi: 10.1093/nar/8.23.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompertz D., Goodey P. A., Thom H., Russell G., Johnston A. W., Mellor D. H., MacLean M. W., Ferguson-Smith M. E., Ferguson-Smith M. A. Prenatal diagnosis and family studies in a case of propionicacidaemia. Clin Genet. 1975 Oct;8(4):244–250. doi: 10.1111/j.1399-0004.1975.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Gravel R. A., Lam K. F., Mahuran D., Kronis A. Purification of human liver propionyl-CoA carboxylase by carbon tetrachloride extraction and monomeric avidin affinity chromatography. Arch Biochem Biophys. 1980 May;201(2):669–673. doi: 10.1016/0003-9861(80)90557-3. [DOI] [PubMed] [Google Scholar]
- Gravel R. A., Lam K. F., Scully K. J., Hsia Y. Genetic complementation of propionyl-CoA carboxylase deficiency in cultured human fibroblasts. Am J Hum Genet. 1977 Jul;29(4):378–388. [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., Darigo M. D., Rosenberg L. E. Isolation and characterization of propionyl-CoA carboxylase from normal human liver. Evidence for a protomeric tetramer of nonidentical subunits. J Biol Chem. 1980 Jan 10;255(1):60–65. [PubMed] [Google Scholar]
- Korneluk R. G., Quan F., Lewis W. H., Guise K. S., Willard H. F., Holmes M. T., Gravel R. A. Isolation of human fibroblast catalase cDNA clones. Sequence of clones derived from spliced and unspliced mRNA. J Biol Chem. 1984 Nov 25;259(22):13819–13823. [PubMed] [Google Scholar]
- Kraus J. P., Williamson C. L., Firgaira F. A., Yang-Feng T. L., Münke M., Francke U., Rosenberg L. E. Cloning and screening with nanogram amounts of immunopurified mRNAs: cDNA cloning and chromosomal mapping of cystathionine beta-synthase and the beta subunit of propionyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2047–2051. doi: 10.1073/pnas.83.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Formation of the 3' end of histone mRNA by post-transcriptional processing. Nature. 1984 Mar 8;308(5955):203–206. doi: 10.1038/308203a0. [DOI] [PubMed] [Google Scholar]
- Lam Hon Wah A. M., Lam K. F., Tsui F., Robinson B., Saunders M. E., Gravel R. A. Assignment of the alpha and beta chains of human propionyl-CoA carboxylase to genetic complementation groups. Am J Hum Genet. 1983 Sep;35(5):889–899. [PMC free article] [PubMed] [Google Scholar]
- O'Dowd B. F., Mahuran D., Cumming D., Lowden J. A. Characterization by nuclear magnetic resonance of the concanavalin A binding oligosaccharide on the beta b chain of placental beta-hexosaminidase B: lectin binding to the separated polypeptide chains of hexosaminidases A and B. Can J Biochem Cell Biol. 1985 Jul;63(7):723–729. doi: 10.1139/o85-090. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Quan F., Willard H. F., Lamhonwah A. M., Korneluk R. G., Lowden J. A., Gravel R. A., Mahuran D. J. Isolation of cDNA clones coding for the beta subunit of human beta-hexosaminidase. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1184–1188. doi: 10.1073/pnas.82.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman L., Weyler W., Shafai T., Young P. E., Nyhan W. L. Prenatal diagnosis of propionic acidemia. JAMA. 1979 Sep 7;242(10):1048–1052. [PubMed] [Google Scholar]
- Tarr G. E., Crabb J. W. Reverse-phase high-performance liquid chromatography of hydrophobic proteins and fragments thereof. Anal Biochem. 1983 May;131(1):99–107. doi: 10.1016/0003-2697(83)90140-9. [DOI] [PubMed] [Google Scholar]
- Upadhyaya M., Archer I. M., Harper P. S., Jasani B., Roberts A., Shaw D. J., Thomas N. S., Williams H. DNA and enzyme studies on chorionic villi for use in antenatal diagnosis. Clin Chim Acta. 1984 Jun 27;140(1):39–46. doi: 10.1016/0009-8981(84)90149-9. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Riordan J. R. Assignment of the gene for myelin proteolipid protein to the X chromosome: implications for X-linked myelin disorders. Science. 1985 Nov 22;230(4728):940–942. doi: 10.1126/science.3840606. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Smith K. D., Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 1983 Apr 11;11(7):2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]