Abstract
Objectives
This study had two objectives: to elicit preferences for current health in a sample of persons with posttraumatic stress disorder (PTSD ) in order to establish quality-of-life estimates for this disorder and to identify symptoms and problems that predict these estimates.
Methods
The authors used the standard gamble (SG), time tradeoff (TTO), and visual analog scale (VAS) methods for quality-of-life estimation at baseline among 184 individuals with chronic PTSD who were participating in a multisite clinical trial. Descriptive statistics were used to characterize quality-of-life estimates for the sample. A linear mixed-effects regression model was conducted to evaluate predictors of quality of life.
Results
The modal participant was a single, white female (77%). The mean±SD age of the sample was 37.31±11.33. On a scale where full health is 1.0 and death is 0.0, mean quality-of-life estimates for living with PTSD were .87±.25, .66±.28, and .64±.20 for SG, TTO, and VAS, respectively. Linear mixed-effects model regression revealed that elicitation method (SG, TTO, and VAS), arousal (a symptom of PTSD), and endorsement of anxiety or depressive symptoms were the strongest predictors of lower quality-of-life scores. Avoidance and re-experiencing of trauma were not predictive of reduced quality of life.
Conclusions
Significant decrements in health-related quality of life were found among persons seeking treatment for PTSD. Although arousal and anxiety and depressive symptoms were predictive of quality-of-life estimates, avoidance and re-experiencing were not. These findings identify targets for symptom resolution that may improve quality of life among persons with PTSD.
Mental health researchers are increasingly interested in costs and outcomes associated with mental health treatments (1). In posttraumatic stress disorder (PTSD), these issues of costs and outcome are paramount; PTSD is more costly than any other anxiety disorder (2). Among the 1.64 million U.S. veterans who have returned from Afghanistan and Iraq, it is estimated that approximately 300,000 individuals currently have PTSD or major depression (3). Direct and indirect costs for these veterans have been estimated at $4.0 to $6.2 billion over two years (4).
In medicine, new treatments are often evaluated in terms of their net monetary benefit, or cost-effectiveness (5). Although in these analyses costs are in monetary units such as dollars, the effectiveness units are typically quality-adjusted life-years (QALYs). QALYs account for both the quantity and quality of life associated with a particular intervention. One year of full health is equal to one QALY. One year of life in a health state less preferable than full health is equal to a fraction of a QALY. For example, if a health state is valued at 3/4, then four years in that health state is equal to 3 QALYs (3/4 × 4 years), where 3/4 is called a quality-of-life estimate.
Quality-of-life estimates for QALY calculation are obtained through preference elicitation exercises that assign numbers to health states consistent with the respondent’s preference. The rates of those health states over time are then observed empirically in studies to determine QALYs gained for any given treatment. For example, the value of major depressive disorder may be determined through preference elicitation exercises describing the condition or by evaluating persons who meet criteria for the disorder. Later in a clinical trial, the aggregate quality of life for different treatment arms may be calculated by assigning different quality-of-life estimates to those with and without the diagnosis after treatment.
The standard gamble (SG), time tradeoff (TTO), and visual analog scale (VAS) are the three most commonly used methods for obtaining quality-of-life estimates. With these approaches, the patient’s current health is summarized via a brief structured interview targeting both broad and disease-specific health domains. Once a current health summary is obtained, preference for staying in one’s current health state is compared with one (or more) alternatives. With the SG method, the alternative health state is a risky choice that will result either in full health or death with some specified probability. With the TTO, the other alternative is full health but for a shortened life span. With the VAS, the other alternatives are full health and death, and the patient is asked to place current health, full health, and death along a continuum of preference. The intervals of placement are assumed to correspond to strength of preference. These methods are described in greater detail below.
Several investigators have established quality-of-life estimates for health states associated with mental health conditions. These have included health states for schizophrenia (6), major depression or dysthymia (7–9), and bipolar disorder (10,11). Freed and colleagues (12) mapped Short Form–36 Health Survey scores for veterans with PTSD to a predicted (community-derived) preference-weighted health status questionnaire score and found reduced quality of life. No studies to our knowledge have elicited health preferences associated with PTSD from actual patients. Yet obtaining such health preferences to establish quality-of-life estimates for PTSD health states is important. In addition, understanding the predictors of quality of life may help direct treatment development.
The purpose of this study was twofold. The first was to elicit preferences for current health among a sample of treatment-seeking persons with chronic PTSD to establish quality-of-life estimates for this disorder. The second purpose was to identify symptoms and problems that predict observed quality-of-life estimates to determine priorities for improving quality of life among persons with PTSD.
Methods
We implemented the SG, TTO, and VAS methods at baseline in a sample of 184 persons who were participating in a multisite clinical trial comparing psychotherapy and pharmacotherapy for the treatment of chronic PTSD. We recruited potential participants through referrals from community clinics, advertisements in the local newspaper, and other advertisements in other media. The study was conducted at two treatment sites in different U.S. regions. After explaining trial procedures and assessments to persons recruited for the clinical trial, we obtained their written informed consent. At each site an institutional review board reviewed and approved this research.
Procedures and assessment
Initial contact with potential participants began with a telephone screening. A semistructured interview was administered with a telephone screening form to elicit eligibility information, such as age, nature of trauma, and the presence of basic inclusion and exclusion criteria. Individuals who screened positive for probable PTSD and met other inclusion criteria were scheduled for a pretreatment assessment. Those who did not meet preliminary criteria were referred for further services as appropriate.
To be included in the study a participant was required to have a current chronic PTSD diagnosis based on DSM-IV criteria, with a minimum duration of 12 weeks since the traumatic event, and to be aged 18 to 65. Exclusion criteria were as follows: a current diagnosis of schizophrenia or delusional disorder; medically unstable bipolar disorder, depression with psychotic features, or depression severe enough to require immediate psychiatric treatment (for example, if the individual was actively suicidal); a current diagnosis of alcohol or substance dependence in the previous three months; an ongoing intimate relationship with the perpetrator (in assault cases); unwillingness to discontinue current psychotherapy or anti-depressant medication (depending on the condition assignment) or discontinuation was not medically advisable; either previous nonresponse or prolonged exposure to sertraline; currently taking sertraline or unwilling to discontinue current trauma-focused psychotherapy; and medical contraindication for the initiation of medication (for example, pregnancy or lactation). Some of the exclusion criteria were implemented because participants were part of an ongoing psychotherapy and medication clinical trial and not for any reason related to the investigation of quality-of-life estimates in this study.
Measures
The PTSD Symptom Scale–Interview Version (PSS-I) (13) was used to determine PTSD diagnostic status. The 17-item PSS-I was validated by using DSM-III-R and DSM-IV symptom criteria. Each symptom is rated on a 0–3 scale of frequency or severity. Paralleling the DSM-III-R, items form three clusters: reexperiencing (four items), avoidance or numbing (seven items), and arousal (six items). Alphas are .85 for the full scale, .69 for the reexperiencing sub-scale, .65 for the avoidance subscale, and .71 for the arousal subscale. Test-retest reliability across one month for the PSS-I was r=.80. Interrater reliability was satisfactory: κ =.91 for diagnosis and r=.97 for symptom severity. The PSS-I, which is administered in 25 minutes, has been validated against the Clinician-Administered PTSD Scale (CAPS) (14), which requires 45 minutes. It was found to have comparable psychometric properties (15).
The Structured Clinical Interview for DSM-IV Axis I and II (SCID) (16) was used to assess whether PTSD was the primary diagnosis. The SCID is a diagnostic interview to acquire information about DSM-IV axes I and II criteria. The SCID I and SCID II have acceptable joint-interview inter-rater reliabilities, with kappas ranging from .70 to .94 (17). In a multisite test-retest reliability study of the SCID-III-R, kappas were .6 or above for current and lifetime diagnoses in major diagnostic categories in patient samples (18).
The SG offers a choice between two alternatives: living at one’s current health status with certainty or taking a gamble between full health with a probability, p, or immediate death with a probability of 1–p. Through a series of two-choice questions in which p varies, the point at which the respondent indicates choices A and B are equivalent is identified. The value of p at the point of equivalence is called the person’s “quality-of-life probability.” Higher quality-of-life probabilities reflect higher utility for the health state being valued. Training and visual aids were used to ensure comprehension of the exercise. The SG technique has been shown to have adequate intrarater reliability (r=.77) (19), as well as satisfactory test-retest reliability (r=.80) (20). Computerized and paper-and-pencil versions of the SG have been validated (21,22). Participants completed a SG via computer using a format provided by Littenberg and colleagues (22).
The TTO is a method of utility assessment developed specifically for the health care setting by Torrance and colleagues (23). Like the standard gamble, the TTO is a two-choice procedure. The participant is asked to choose the more preferable of two options: choice A is x years in full health, and choice B is t years at his or her current health status (x > t for all questions). While t is held constant across questions, the value of x changes with each successive question. Choices are made until an indifference point is reached. At indifference the ratio x/t, or proportional time tradeoff, represents the value of current health relative to full health and death. The TTO technique has been shown to have adequate intrarater reliability (range .77–.88) (19,24), as well as satisfactory test-retest reliability (r=.87) (20).
The VAS method provides a simple technique for assigning numerical values to current health. Typically, the respondent is shown a “feeling thermometer” that is 100 units in length, with well-defined end points. The task is to mark the line to indicate where his or her preference rests in relation to the two poles. The respondent is also asked to mark the line to indicate where his or her preference rests for perfect health and death. The value of current health can then be expressed as a simple linear transformation of the scale values. The VAS method has been shown to have adequate intrarater reliability (range .74–.94) (24), as well as satisfactory test-retest reliability (r=.77) (20).
Participants were asked to complete a current health summary, which included domains and levels of dysfunction (Table 1). The summary consisted of four domains, each having three levels, and described 34=81 different current health states associated with PTSD. The current health summary tool was developed with use of guidelines from the U.S. Panel on Cost-Effectiveness (25) and with consideration of symptoms of PTSD described in the DSM-IV (26).
Table 1.
Current health summary domains and levels of dysfunction completed by 184 persons with chronic posttraumatic stress disorder
| Domain | Level of dysfunction |
|---|---|
| Reexperiencing |
|
| Avoidance |
|
| Arousal |
|
| Anxiety and depression |
|
Statistical analysis
All statistical analyses were completed in R (27). We report box plots of SG, TTO, and VAS values for the sample. In addition, we report results for symptoms and problems that predict lower health utility (lower quality of life). To obtain these results, we conducted linear mixed-effects model analysis of symptom-problem endorsement and its effect on quality-of-life score (28). Mixed-effects models (or mixed models) include random-effect terms in addition to the usual error term and are appropriate for modeling responses when data are nested in a hierarchical structure. In our case, observations were taken for three approaches to assessing a health utility (SG, TTO, and VAS), and responses (health utilities) are nested within elicitation method. In our analysis, we estimated fixed effects for elicitation procedure (SG, TTO, and VAS), as well as endorsement of symptoms of PTSD, anxiety, and depression. We also estimated random effects for elicitation method and participant. The mixed-model approach provides a regression equation that predicts health utility. Further, the results can be used by other investigators to estimate health utility in cost-effectiveness models.
Results
Of the 184 participants, 141 (76%) were female; 125 (68%) were white, 36 (20%) were African American, ten (5%) were Asian, three (2%) were Native American or Alaskan Native, one (<1%) was native Hawaiian or Pacific Islander, and nine (5%) endorsed the category “other.” The mean±SD age of the sample was 37.31±11.33.
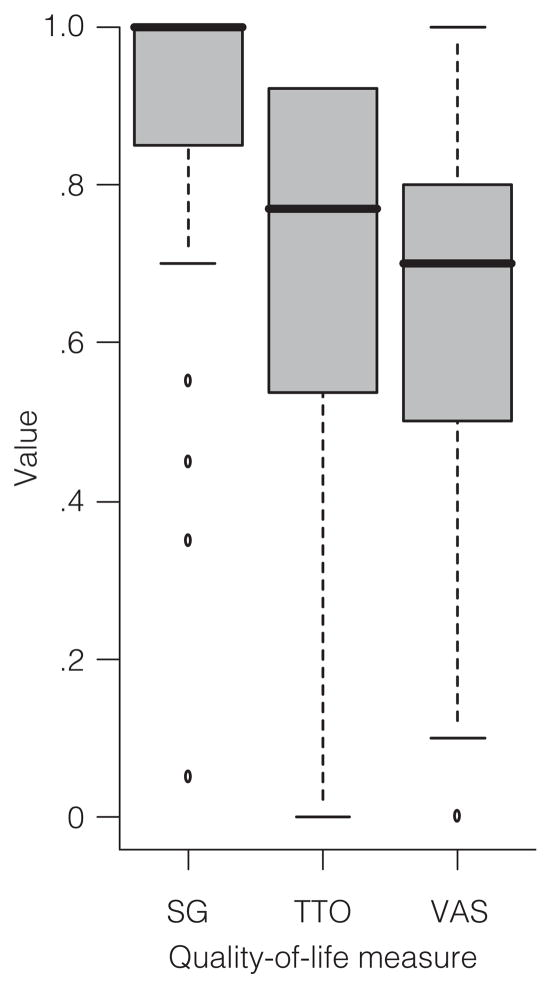
With full health valued at 1.0 and death valued at 0.0, the mean current health utility scores for persons with a PTSD diagnosis were .87±.25, .66± .28, and .64±.20 for SG, TTO, and VAS, respectively. Figure 1 illustrates a box plot of SG, TTO, and VAS scores for the 184 participants. As is clear, SG scores were the highest, followed by TTO scores and VAS scores.
Figure 1.
Box plots for the standard gamble (SG), time tradeoff (TTO), and visual analog scale (VAS) used for measuring quality of life of 184 persons with chronic posttraumatic stress disordera
a Full health=1.0; death=0.0.
Because the SG method involves risk (of death), risk aversion is thought to explain the elevation of SG scores relative to TTO and VAS scores (28). However, the vast majority of participants were willing to bear at least some risk of death (at least one chance in 10,000) to achieve better health.
Table 2 illustrates the linear mixed-effects model regression weights and significance levels for endorsement of symptoms of PTSD and anxiety or depression in predicting preference for health, as represented by SG, TTO, and VAS scores. The mixed-effects regression estimated health utility as a function of elicitation method, reexperiencing of trauma, avoidance, arousal, and symptoms of anxiety or depression.
Table 2.
Mixed-effects model of predictors of health preferences among 184 persons with chronic posttraumatic stress disordera
| Variable | Estimate | SE | df | t |
|---|---|---|---|---|
| Intercept | .82 | .07 | 11.42*** | 262 |
| Quality-of-life measurea | .11 | .01 | 8.21*** | 262 |
| Symptomb | ||||
| Reexperiencing | −.03 | .03 | −1.18 | 148 |
| Avoidance | −.03 | .03 | −.99 | 148 |
| Arousal | −.04 | .02 | −1.65* | 148 |
| Anxiety or depression | −.05 | .02 | −1.94** | 148 |
Measure: 1=visual analog scale; 2=time tradeoff; 3=standard gamble
Problem: 1=none, 2=some, 3=major
p<.05
p<.01
p<.001
The elicitation method (SG, TTO, and VAS), PTSD arousal, and endorsement of anxiety and depressive symptoms were all predictive of health utility (Table 2). In contrast, reexperiencing of trauma and avoidance did not explain decreases in quality of life.
Table 3 illustrates the correlations between mixed-model variables and the model intercept. Symptom endorsements were mildly correlated with each other and, as expected, un-correlated with elicitation method.
Table 3.
Correlations between mixed-model variables and the model intercept for predictors of health preferences among 184 persons with chronic posttraumatic stress disorder
| Variable | Intercept | Quality-of-life measure | Reexperiencing | Avoidance | Arousal |
|---|---|---|---|---|---|
| Quality-of-life measure | −.352 | ||||
| Reexperiencing | −.171 | −.009 | |||
| Avoidance | −.351 | −.005 | −.310 | ||
| Arousal | −.341 | .013 | −.355 | −.027 | |
| Anxiety and depression | −.244 | .015 | −.149 | −.202 | −.231 |
Discussion
Our results suggest significant decrements in health-related quality of life among persons with chronic PTSD. We measured quality of life in terms of tradeoffs in risk of death (SG), duration of survival (TTO), and strength of preference (VAS). The mean SG score of .87 indicates that the average patient was willing to accept a treatment with a 13% chance of immediate death in order to achieve total relief of his or her PTSD symptoms and problems. Assuming, for example, a 77-year life span for the average participant, the observed mean score of .66 on the TTO indicates that the average patient contemplating a long life with PTSD symptoms was willing to trade (give up) 13.6 years of life to live his or her remaining life span unburdened by these symptoms (that is, to live a life in full health). Strength of preference estimates via the VAS were close to TTO values. The scores by all three methods were generally in the range of those for serious medical problems, such as stroke, heart attack, and cancer (30). Furthermore, quality-of-life scores are similar to those for major depression but slightly higher than for disorders involving psychoses (bipolar disorder and schizophrenia). In a comprehensive review paper, Tengs and Wallace (30) reported median quality-of-life scores for an episode of depression and of severe bipolar disorder or schizophrenia of .77 and .65, respectively.
Clearly, SG scores were higher than TTO and VAS scores. Bleichrodt and colleagues (31) and Doctor and colleagues (32) have found that the SG may be susceptible to cognitive bias that may inflate SG scores. These authors offered correction formulas that tend to make SG values more similar to TTO and VAS values. However, the mixed-effects model approach that we used accounts for any SG bias in its estimation of random effect associated with elicitation method. Results from the mixed-effects model may be used to estimate utility in future PTSD studies when only symptom and problem endorsement is available. Should researchers want to use a single measure, the TTO is generally viewed as providing the least biased estimates of quality of life and is less burdensome cognitively than the SG and only slightly more difficult than the VAS (32,33). Reliability among the three measures is comparable.
It is interesting that we found only a subset of PTSD symptoms to be associated with lower quality of life. Specifically, arousal and more general anxiety or depressive symptoms were associated with lower current health quality-of-life estimates. In contrast, reexperiencing of trauma and trauma-related avoidance were not associated with lower quality of life. Because the patient had to retain the information from the health summary to complete preference exercises, these summaries had to be brief. Thus symptoms of anxiety and depression were represented in an inclusive-disjunctive form (that is, A or B or both). Therefore, we cannot disentangle the separate effects of anxiety and depressive symptoms on preference in our study and must treat them as a group. The effect of depressive symptoms on quality of life is well documented (7–9). Anxiety may have a strong effect in lowering health preference, and the study presented here is one of the first to document decrements in quality-of-life estimates elicited from patients with PTSD. Other studies have evaluated preferences for anxiety states among general population samples (34). Different elicitation methods produced different quality-of-life values, but systematic differences among methods were captured by our mixed-model approach. This allowed use of symptoms and problems as predictors to explain variance in preference common to the three elicitation techniques.
Arousal may be associated with general feelings of discomfort that reduce quality of life. We conjecture that although avoidance is an important aspect of PTSD, its effect on quality of life may be limited because it is a coping strategy to decrease subjective discomfort. Thus, although it interferes with long-term objectives of the patient, avoidance may be a dysfunction that improves, in the short term, subjective well-being. We are uncertain as to why reexperiencing was not associated strongly with lower quality of life. One possibility is that reexperiencing is mediated by avoidance: the more a person is able to avoid the trauma the less he or she is confronted with unwanted reexperiencing of it. If this is the case, persons coping by means of avoidance may not reexperience traumatic events as often as those who do not cope by avoidance.
The relationship between quality-of-life estimates by persons with PTSD and by persons in the general population is not known. For medical conditions, preference scores are generally higher for persons with the disorder (24). However, a recent study by Pyne and colleagues (35) found the opposite result for depression: persons with a diagnosis of depression reported lower quality-of-life scores than persons in the general population.
There are several limitations to this study that deserve mention. First, and as noted above, symptoms of anxiety and depression were endorsed in a single item, and the individual effects cannot be disentangled. Second, the study involved elicited preferences, which may differ from preferences reflected in everyday decision making. However, the latter is difficult to measure in field studies, because persons with PTSD are not often faced with the types of tradeoffs that are necessary for calculating quality-of-life estimates. Third, participants were drawn from a treatment-seeking sample, which was a mix of referrals from professionals, referrals from community agencies, and self-referrals—either those who found out about the study on their own or those recruited from advertisements. Thus the sampling method did not include some persons with a PTSD diagnosis who were not seeking treatment.
Conclusions
The data presented here provide quality-of-life estimates useful for cost- and outcomes-modeling studies. Further, among individuals with chronic PTSD, severity of general anxiety and depressive symptoms and arousal were associated with lower quality of life. The relationship between avoidance and quality of life may be complicated by the ability of avoidance to reduce subjective discomfort in the short term. Future studies should evaluate the mechanism by which symptoms and problems affect quality of life.
Acknowledgments
This research was made possible by grants R01MH066347 and R01MH066348 from the National Institute of Mental Health (“Effectiveness of PTSD Treatment: Prolonged Exposure Therapy vs. Zoloft”).
Footnotes
Disclosures
The authors report no competing interests.
Contributor Information
Dr. Jason N. Doctor, Email: jdoctor@usc.edu, Research faculty at the Leonard D. Schaeffer Center for Health Policy and Economics and is associate professor in the School of Pharmacy, University of Southern California, 1540 East Alcazar St., CHP140, Los Angeles, CA 90089
Dr. Lori A. Zoellner, Director of the Center for Anxiety and Traumatic Stress and is associate professor in the Department of Psychology, University of Washington, Seattle
Dr. Norah C. Feeny, Associate professor in the Department of Psychology, Case Western Reserve University, Cleveland, Ohio
References
- 1.Suh GH, Han C. Mental health economics, health service provision, and the practice of geriatric psychiatry. Current Opinion in Psychiatry. 2008;21:546–550. doi: 10.1097/YCO.0b013e32830f1bd5. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, Sistsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990’s. Journal of Clinical Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 3.Tanielian T, Jaycox LH. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Washington, DC: RAND; 2008. [Google Scholar]
- 4.Eibner C, Ringel JS, Kilmer B, et al. The cost of post-deployment mental health and cognitive conditions. In: Tanielian T, Jaycox LH, editors. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Washington, DC: RAND; 2008. [Google Scholar]
- 5.Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annual Review of Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 6.Briggs AH, Wild D, Lees M, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Quality Life Outcomes. 2008;6:105. doi: 10.1186/1477-7525-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett KJ, Torrance GW, Boyle MH, et al. Cost-utility analysis in depression: the McSad utility measure for depression health states. Psychiatric Services. 2000;51:1171–1176. doi: 10.1176/appi.ps.51.9.1171. [DOI] [PubMed] [Google Scholar]
- 8.Schaffer A, Levitt AJ, Hershkop SK, Oh P, et al. Utility scores of symptom profiles in major depression. Psychiatry Research. 2002;110:189–197. doi: 10.1016/s0165-1781(02)00097-5. [DOI] [PubMed] [Google Scholar]
- 9.Revicki DA, Wood M. Patient-assigned health state utilities for depression-related outcomes: differences by depression severity and antidepressant medications. Journal of Affective Disorders. 1998;48:25–36. doi: 10.1016/s0165-0327(97)00117-1. [DOI] [PubMed] [Google Scholar]
- 10.Revicki DA, Hanlon J, Martin S, et al. Patient-based utilities for bipolar disorder–related health states. Journal of Affective Disorders. 2005;87:203–210. doi: 10.1016/j.jad.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Tsevat J, Keck PE, Hornung RW, et al. Health values of patients with bipolar disorder. Quality of Life Research. 2000;9:579–586. doi: 10.1023/a:1008979825704. [DOI] [PubMed] [Google Scholar]
- 12.Freed MC, Yeager DE, Liu Xian, et al. Preference-weighted health status of PTSD among veterans: an outcome for cost-effectiveness analysis using clinical data. Psychiatric Services. 2009;60:1230–1238. doi: 10.1176/ps.2009.60.9.1230. [DOI] [PubMed] [Google Scholar]
- 13.Foa EB, Riggs DS, Dancu CV, et al. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–473. [Google Scholar]
- 14.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 15.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale–Interview Version and the Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Williams JBW, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R (SCID I): history, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 17.Skre I, Onstad S, Torgeson S, et al. High interrater reliability for the Structured Clinical Interview for DSM-III-R Axis I (SCID-1) Acta Psychiatrica Scandinavica. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams J, Gibbon M, First M, et al. The Structured Clinical Interview for DSM-III-R (SCID) II: multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 19.Torrance GW. Social preferences for health states: an empirical evaluation of three measurement techniques. Socioeconomic Planning Sciences. 1976;10:129–136. [Google Scholar]
- 20.O’Connor AM, Boyd NF, Till JE. Influence of Elicitation Technique, Position Order and Test-Retest Error on Preferences for Alternative Cancer Drug Therapy. Proceedings of the 10th National Nursing Research Conference; Toronto, Ontario: University of Toronto; 1985. [Google Scholar]
- 21.Lenert LA, Sturley A, Watson ME. iM-PACT3: Internet-based development and administration of utility elicitation protocols. Medical Decision Making. 2002;22:464–474. doi: 10.1177/0272989X02238296. [DOI] [PubMed] [Google Scholar]
- 22.Littenberg B, Partilo S, Licata A, et al. Paper Standard Gamble: the reliability of a paper questionnaire to assess utility. Medical Decision Making. 2003;23:480–488. doi: 10.1177/0272989X03259817. [DOI] [PubMed] [Google Scholar]
- 23.Torrance GW, Thomas WH, Sackett DL. A utility maximization model for evaluation of health care programs. Health Services Research. 1972;2:118–133. [PMC free article] [PubMed] [Google Scholar]
- 24.Torrance GW. Preferences for health states: a review of measurement methods. Mead Johnson Symposium on Perinatal and Developmental Medicine. 1982;20:37–45. [PubMed] [Google Scholar]
- 25.Gold MR, Patrick DL, Torrance GW, et al. In: Identifying and valuing outcomes; in Cost-Effectiveness in Health and Medicine. Gold MR, Siegel JE, Russell LB, et al., editors. Oxford, United Kingdom: Oxford University Press; 1996. [Google Scholar]
- 26.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 27.Ihaka R, Gentleman R. R: a language for data analysis and graphics. Journal of Computational and Graphical Statistics. 1996;5:299–314. [Google Scholar]
- 28.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S- PLUS. New York: Springer; 2000. [Google Scholar]
- 29.Weinstein MC, Siegel JC, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 30.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Medical Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Bleichrodt H, Pinto JL, Wakker PP. Making descriptive use of prospect theory to improve the prescriptive use of expected utility. Management Science. 2001;47:1498–1514. [Google Scholar]
- 32.Doctor JN, Bleichrodt H, Lin HJ. Health utility bias: a systematic review and meta-analytic evaluation. Medical Decision Making. 2010;30:58–67. doi: 10.1177/0272989X07312478. [DOI] [PubMed] [Google Scholar]
- 33.Bleichrodt H. A new explanation for the difference between time trade-off utilities and standard gamble utilities. Health Economics. 2002;11:447–456. doi: 10.1002/hec.688. [DOI] [PubMed] [Google Scholar]
- 34.Dolan P. Modeling valuations for EuroQol health states. Medical Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Pyne JM, Fortney JC, Tripathi S, et al. How bad is depression? Preference score estimates from depressed patients and the general population. Health Services Research. 2009;44:1406–1423. doi: 10.1111/j.1475-6773.2009.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]