Abstract
Dyskeratosis congenita (DC) was originally defined as a rare inherited bone marrow failure syndrome associated with distinct mucocutaneous features. Today DC is defined by its pathogenetic mechanism and mutations in components of the telomere maintenance machinery resulting in excessively short telomeres in highly proliferating tissues. With this new definition the disease spectrum has broadened and ranges from intrauterine growth retardation, cerebellar hypoplasia, and death in early childhood to asymptomatic mutation carriers whose descendants are predisposed to malignancy, bone marrow failure, or pulmonary disease. The degree of telomere dysfunction is the major determinant of disease onset and manifestations.
Keywords: dyskeratosis congenita, telomerase, pulmonary fibrosis, aplastic anemia, anticipation, telomere
Introduction
Dyskeratosis congenita (DC) is a rare inherited bone marrow failure disorder that has garnered much attention in the last decade due to its unique pathogenesis. DC is caused by defects in telomere maintenance. Telomeres are complex DNA-protein structures that protect the chromosome ends from degradation and inappropriate recombination and thus are of critical importance to the cell and the living organism. When the Nobel Prize in Medicine was awarded to Elizabeth H. Blackburn, Carol W. Greider, and Jack W. Szostak in 2009 for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase, the orphan disease DC, once an obscure curiosity in pediatric hematology, moved into the limelight of biomedical research. The insights gained by studying telomere biology have dramatically changed how clinicians diagnose and assess patients and their families with DC. In turn, the study of patients and families with DC has allowed investigators to delineate the consequences of dysfunctional telomere maintenance in humans [1]
Telomeres, telomerase, and human disease
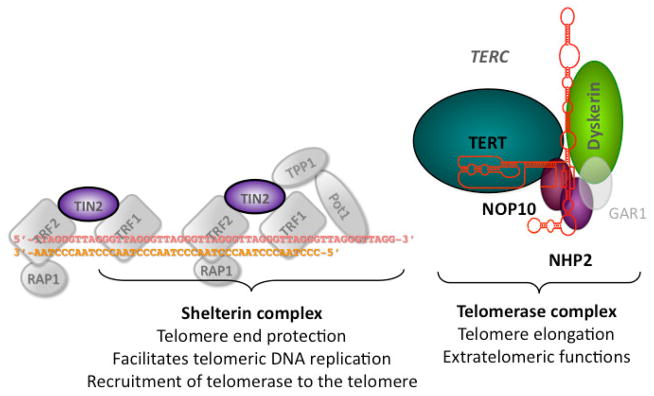
Several excellent reviews have recently summarized our current knowledge of the structure and function of telomeres and telomerase [2,3]. Telomeres are complex DNA-protein structures at the ends of chromosomes (see Fig. 1A [4,5]). In most eukaryotes including humans, telomeric DNA is composed of guanine-rich DNA repeat sequences [6]. Telomeres shorten with each cell division [7]. When telomeres become critically short a DNA damage response is activated causing cell cycle arrest and senescence or cell death [8]. In quiescent eukaryotic cells the single-stranded 3′ overhang folds back on the double-stranded telomere forming the t-loop structure, which serves to protect the chromosome terminus. Shelterin, a complex of six proteins [telomeric repeat binding factor 1 and 2 (TRF1, TRF2), TRF1-interacting nuclear factor (TIN2), protection of telomeres 1 (POT1), TPP1, and RAP1], binds to telomereic DNA, protects the telomere structure, facilitates telomeric DNA replication, and is required for the recruitment of telomerase to the telomere end (Fig. 1) [9].
Figure 1.
Diagram of the telomere ends and of telomerase and the components mutated in patients with Dyskeratosis Congenita. The 6 proteins of the shelterin complex (TRF1, TRF2, RAP1, TIN2, TPP1, and POT1) protect the telomere from being recognized as a double strand break and regulate the access of telomerase. Telomerase is a ribonucleoprotein consisting of the catalytic subunit (TERT), its RNA component TERC, and the four H/ACA RNA associated proteins dyskerin, NHP2, NOP10 and GAR1. The 6 genes known to be mutated in patients with DC are highlighted in color. No mutations have been indentified in GAR1, or the other components of the shelterin complex.
In humans telomerase-based telomere elongation is the major mechanism that counteracts the process of continuous telomere shortening [10,11]. The telomerase enzyme is a ribonucleoprotein complex consisting of the catalytic subunit TERT, its RNA component TERC, and the four H/ACA RNA associated proteins dyskerin, NHP2, NOP10 and GAR1 (see Fig. 1) [12,13]. Telomerase synthesizes telomeric repeat sequences onto the telomeres. Telomerase is also thought to have extra-telomeric functions important in stem cell proliferation [14]. In humans after birth telomerase expression is confined to germ cells, stem cells and their immediate progeny, activated T cells, and monocytes: all other somatic cells lack telomerase activity or express telomerase only at very low levels. Overexpression immortalizes primary human cells from many tissues [15] and telomerase activation plays an important role in tumor progression and tumor maintenance (reviewed in Shay et al. 2001 [16]). Notably, most cancer cells express telomerase activity [8,16]. Despite telomerase expression in stem cells, human telomeres shorten with age [17,18]. In peripheral white blood cells rapid telomere shortening occurs within the first year of life, followed by a more gradual decline over time[19]. Although there is considerable variation among individuals of the same age, the relative telomere length is at least in part inherited [20]. Telomere shortening has been implicated in the mechanism of aging, and more recently it has been shown to increase the risk of heart disease and of the development of lung cancer (for review see Harley 2005 [21]).
Dyskeratosis congenita
DC was first described over 100 years ago and was defined by the association of three clinical features: dystrophic nails, oral leukoplakia (white spots on the tongue and oral mucosa), and abnormal skin pigmentation [22]. While each of these hallmarks may be seen in other clinical conditions, the presence of all three is pathognomonic of DC. The association of DC with bone marrow failure (BMF), malignancy, and pulmonary fibrosis (PF) was recognized later. Historically, the diagnosis of DC in a patient with BMF or PF required the presence of at least one of the three classic mucocutaneous features (for review see [1]). Other somatic abnormalities have been described in some patients with DC including epiphora (excessive tears due to the obstruction of the tear ducts), early graying of the hair, premature tooth loss, enteropathy with malabsorption, immune deficiency, esophageal stricture, cadiomyopathy, liver cirrhosis, osteoporosis and avascular necrosis of the bone, urinary tract abnormalities, testicular atrophy, eye abnormalities, mental retardation, intracranial calcifications and cerebellar hypoplasia with ataxia [1,23]. Many of these symptoms are not present at birth but may develop in childhood, adolescence, or later in life. Most importantly, patients with DC have a predisposition for the development of a variety of malignancies, in particular myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML), head/neck cancer, and esophageal cancer [24,25]. Malignancies tend to occur earlier in life compared to the same malignancies in non-DC individuals and are often the cause of death in patients in the third, fourth and fifth decade of life. Individuals with DC may develop independent tumors at more than one site [25,26].
DC gene mutations and the association with telomere maintenance
The understanding that DC is caused by defects in telomere maintenance came with the positional cloning of the gene responsible for the X-linked form of the disease, DKC1, encoding the protein dyskerin [27], and the subsequent demonstration that dyskerin is an essential component of the telomerase complex necessary for the stabilization of telomerase RNA [28,29]. The association of DC with defective telomere maintenance was confirmed by the finding that the dominant form of the disease could be caused by mutations in other components of telomerase, the telomerase RNA [30] TERC and reverse transcriptase TERT [31]. In some families in which DC is inherited in a recessive manner mutations in NOP10 [32] and NHP2 [33] have been shown to be responsible for the disease. These two proteins associate with dyskerin and all three proteins are associated with both the telomerase complex and with H/ACA snoRNPs, nucleolar complexes that catalyze site-specific pseudouridylation of nascent ribosomal RNA (see Fig. 1). Whether ribosomal RNA processing is affected in X-linked or recessive forms of DC and contributes to the pathology is controversial, though it is generally accepted that the major pathogenic features relate to defective telomere maintenance [34,35]. The sixth DC gene to be identified, TINF2, was found in about 10% of clinically diagnosed DC cases, including many with an early disease onset and a severe clinical presentation [36]. This gene encodes a protein, TIN2, that is not in the telomerase complex but is a component of the protein complex, shelterin, which binds to telomere DNA repeats, protecting them from degradation and from DNA repair enzymes [9]. How TIN2 mutations cause DC is not yet fully understood. While the mechanism may involve deprotection of the telomere ends, we favor the hypothesis that mutations in TIN2 impair the access of telomerase to the telomere end. TERC RNA levels and presumably the enzymatic activity of telomerase are not altered in patients with TINF2 mutations [37]. Furthermore, impaired activity of the telomerase at the telomere end also nicely explains the phenotypic similarity of patients with the mutations in the telomerase complex (DKC1, TERC, TERT, NOP10, NHP2) and patients with mutations in TIN2 residing at the telomere.
In about 50% of patients with the classic features of DC the pathogenic mutations have not yet been identified, suggesting that additional gene products are involved in the same pathway of telomere maintenance. Notably, no mutations have been identified so far in the genes encoding the other five shelterin-associated proteins (TRF1, TRF2, RAP1, POT1, and TPP1).
Variable disease penetrance and expressivity
With the knowledge of the genes mutated in DC, genetic testing became available for family members and for individuals presenting similar symptoms but lacking the classic diagnostic features. Examination of kindreds revealed that TERC or TERT gene mutations that caused DC with the classic mucocutaneous features in some family members were silent in others (variable penetrance). In some family members these mutations caused only isolated clinical features, such as BMF [31], PF [38,39], liver cirrhosis [40], osteoporosis [41] or MDS [42] (variable expressivity).
Whether patients without the typical mucocutaneous features or silent mutations carriers should be labeled with DC is controversial. Alternative names have been proposed such as “syndromes of telomere shortening” or telomeropathies [43,44]. However, there are other disorders that are associated with telomere dysfunction and telomere shortening such as Werner’s syndrome - a premature aging syndrome caused by mutations in WRN, an ATP-dependent helicase, [45] or the Seckel syndrome, a recessive syndrome of growth retardation and microcephaly due to mutations in ATR [46–48]. Although these disorders also affect the telomeres and are associated with telomere shortening in certain cells or tissues, they are clearly clinically as well as pathogenetically distinct from DC. Historically it has proven difficult and confusing to change the name of a disease; thus “atypical” and “silent” DC or “asymptomatic mutation carrier” might be better terms for classifying individuals with disease but without the classic mucocutaneous features or mutation carriers with no clinical detectable disease. The finding that in some family members DC may present only with one of the clinical features associated with DC led to the genetic screening of patient populations whose primary and only presentation was that of BMF, PF, or liver cirrhosis and indeed a small (2–10%) but distinct population in each was found to have disease due to a mutation in a DC associated gene. [38–40,49,50]. Although it is often written that mutations in DC associated genes may cause different diseases such as BMF, PF, or liver cirrhosis, these conditions have to be understood as different clinical presentations of one and the same disease (i.e., DC), and that different clinical presentations may be found within an individual family carrying the identical gene mutation. Frequently laboratory signs of additional organ dysfunction may be present even in the absence of a clinical disease phenotype (e.g., even when peripheral blood cell counts are normal, the bone marrow may be hypocellular and lack the ability to reconstitute hematopoiesis when used for hematopoietic stem cell transplantation), or symptoms may develop later in life (e.g., the development of PF or liver cirrhosis in patients with DC after successful hematopoietic stem cell transplantation). In some families disease may only occur under certain circumstances (e.g., BMF may only occur after a severe infection or after chemo- or radiation therapy for cancer, or PF may arise only in the setting of tobacco smoking).
Inheritance of DC
In the pre-molecular diagnostic area patients with DC were classified according to the inheritance pattern of disease as X-linked, autosomal dominant, or autosomal recessive DC, assuming that distinct genetic defects would account for the different inheritance patterns (see also Fig. 3). However, the identification of genes mutated in patients with DC and the availability of clinical genetic testing have shown that the inheritance of disease in patients with DC may be more complex [41]. Mutations in DC associated genes may occur sporadically due to de novo gene mutations. In fact the alanine to valine mutation at position amino acid 353 (A353V) in dyskerin accounting for about 40% of the DKC1 mutations [51] frequently occurs as a de novo mutation and so do the majority of cases of the R282H and R282C mutations in TINF2. In contrast, mutations in the TERT and TERC genes are usually inherited, with the exception of G178A and C180T mutations in TERC reported to be present only in the patient but not in either parent [52]. Mutations in the X-linked DKC1 gene almost always cause disease in males, even in the first generation the mutations occurs. In contrast, female mutation carriers rarely show disease manifestations because most tissues only express the normal DKC1 allele due to biased X chromosome inactivation. Interestingly the Y139H, V126M and X154Arg NHP2 mutations [33] and the R34W NOP10 mutation [32] cause disease when homozygous or (in the case of NHP2 V126M and X154R) compound heterozygous. The most likely explanation is that these are hypomorphic mutations, which do not, when present in the heterozygous state, sufficiently impair telomerase activity to cause notable disease. Nevertheless telomeres in the heterozygous parents were shortened compared to normal controls, which is consistent with this hypothesis. Homozygosity or compound heterozygosity have also been described for patients carrying disease and TERT or TERC gene mutations [41,53,54] showing recessive inheritance in the affected family. Interestingly homozygous or compound heterozygous TERT or TERC gene mutation carriers often have more severe disease compared to heterozygous mutation carriers and not uncommonly present with the classical features of DC (see also Fig. 4). However, in contrast to mice homozygosity or compound heterozygosity for telomerase null mutations have not been described in humans, suggesting that these are incompatible with life in humans. Similarly no DKC1 null mutation has been described in patients with DC. This might be due to the essential role of dyskerin in ribosome biogenesis, but could also be due to its role in telomere maintenance, as pathogenic DKC1 mutations may greatly diminish but not completely abolish the accumulation of telomerase RNA, TERC, in the cell.
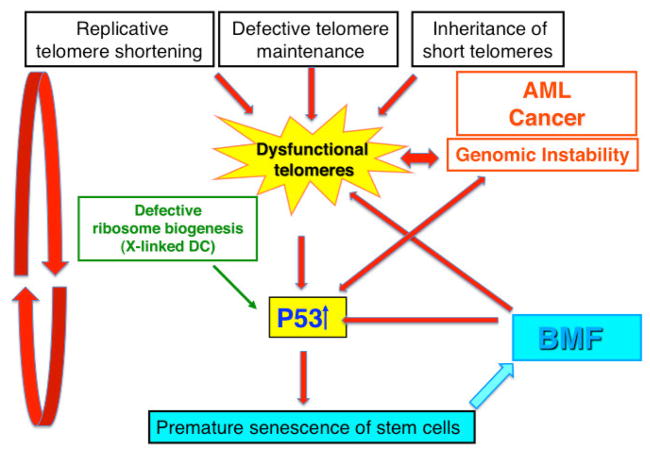
Figure 3.
Model of the pathogenesis of BMF and malignancy in patients with DC. Our hypothesis is that dysfunctional telomeres play a central role in the pathogenesis of BMF and malignancy in patients with DC. Dysfunctional telomeres cause the activation of the p53 pathway leading to cell cycle arrest, senescence, and cell death in replicating progenitor cells. Depletion of proliferating progenitor cells recruits new stem cells into cell cycle, thus initiating a vicious cycle that ultimately leads to the depletion of stem cells and to the clinical picture of AA. Increased oxidative stress in the environment of BMF and defective ribosome biogenesis in the X-linked and some rare autosomal recessive forms of DC may additionally contribute to the activation of the p53 pathway. Dysfunctional telomeres cause genomic instability that leads to cancer predisposition in patients with DC.
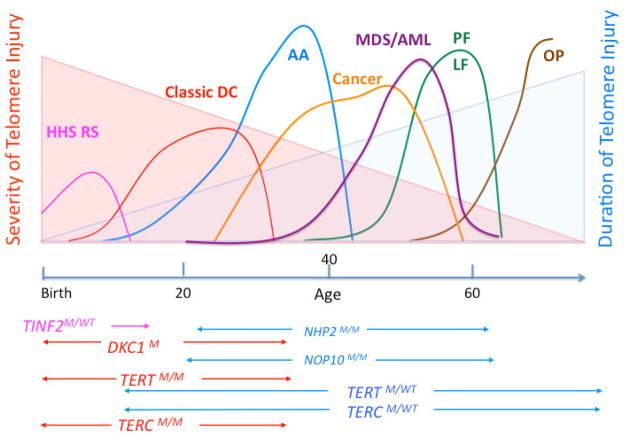
Figure 4.
Age-related variable disease expression of mutations in DC associated genes.
The clinical phenotype of mutations in DC related genes varies with the age of onset. Mutations that most severely compromise telomere maintenance manifest early in life leading to the more severe forms of DC including the HHS (Hoyeraal Hreidarrson syndrome) or RS (Revesz syndrome) They may be caused by mutations in the DKC1 and TINF2 gene. Classic DC is most frequently caused by DCK1 gene mutations or homozygous or compound heterozygous mutations in the TERC or TERT genes. Heterozygous mutations in TERC or TERT genes may be associated with BMF, whereas mucco-cutaneous features may be mild or missing (aplastic anemia, AA). Pulmonary fibrosis (PF) or liver fibrosis (LF) are DC manifestations that clinically become obvious later in life. They may present as the only clinical feature in patients heterozygous for TERT or TERC gene mutations. Malignancy (cancer and MDS/AML) is most frequently seen in patients with mild-moderate disease severity and occurs most frequently in the third, fourth and fifth decade. In patients with TERT or TERC gene mutations, MDS or MDS/AML might be the initial presentation at diagnosis. The severity and the duration of dysfunctional telomere maintenance are important factors that determine the disease pathology. M=mutant.
Anticipation in DC
DC shows a unique and previously unknown form of disease anticipation. Disease anticipation is an increase in disease severity in later generations carrying a gene mutation. Most human diseases showing anticipation are neurological conditions caused by repeated trinucleotide sequences in a gene [55]. These are often CAG repeats and encode polyglutamine in the mature protein, though in some cases they are other sequences and can be in non-protein encoding regions. These repeats can expand by unequal crossing over during meiosis and can increase in length as they are passed from generation to generation. Classic examples are Huntington’s disease [56] and the fragile X mental retardation syndrome [57]. Anticipation in DC has a different mechanism – it is due to telomere shortening and the inheritance of shortened telomeres, such that early generations carrying a TERC or TERT mutation may be unaffected, but in later generations telomeres become critically short and affect the ability of stem cells to continually renew tissues with a high cellular turnover. Initially anticipation was described in telomerase null mice who only develop a disease phenotype after several rounds of inbreeding, and experiments in mice have elegantly shown that this is due to the inheritance of subsequently shorter telomeres with each round of inbreeding [58,59]. Anticipation has also been documented in certain families with the autosomal dominant form of DC [60–62]. These families show increased severity of disease and earlier age of onset in later generations. Anticipation in the X-linked form is more difficult to observe, most likely because in females, due to X-inactivation in early embryogenesis and the subsequent selective growth advantage of cells expressing the normal DKC1 allele, the mutated DKC1 gene is usually on the inactive X-chromosome and thus is not expressed. Thus, when the DKC1 gene mutation is alternately passed through females (male to male inheritance does not occur for X-linked DC) telomere length is usually restored at least to some degree. Whether disease anticipation may be observed between grandfather and grandson is hard to determine because of the rarity of the disease and the fact that X-linked DC is often associated with decreased male fertility.
Mutations in DC associated genes
Sequence alterations in DC associated genes are summarized in the Telomerase Database (http://telomerase.asu.edu/diseases.html) along with references to the original work. In X-linked DC, with the exception of a small terminal deletion removing the last 20 amino acids of dyskerin and an in-frame deletion of a single amino acid (L37) mutations in dyskerin are mainly point mutations causing a single amino acid change, suggesting that these are all hypomorphic mutations with some residual dyskerin function (see also above). The promoter and splice site mutations described in rare patients with DC are consistent with this interpretation. The solving of the crystal structure of dyskerin revealed that pathogenic DKC1 mutations cluster in the RNA binding domain of dyskerin [63–65], which is consistent with the decreased TERC RNA levels in dyskerin mutant cells. Large and small TERC RNA deletions have been described to be responsible for the development of autosomal dominant DC. Point mutations alter the tertiary structure of the RNA affecting activity or processivity of the telomerase enzyme, or the stability of the RNA. Mutations in TERT spread throughout the coding sequence and may affect any of its three domains, the N-terminal region, reverse-transcriptase motifs, or the C-terminal region. The study of 45 individuals with a de novo chromosomal deletion spanning the TERT gene showed that telomere shortening, although marginally accelerated, was not sufficient to cause a DC-related disease in the absence of anticipation [66]. Alternatively, it is possible that in patients with a gene deletion compensatory mechanisms exist that might not operate in patients who express a mutant protein. Interestingly in patients with DC due to TINF2 mutations the mutations cluster in exon 6 [36,37]. The mutations spare the TRF1 binding site, with TIN2 mutations after the binding site giving rise to a more severe phenotype (see also below).
The majority of mutations in patients with DC are unique or private mutation occurring in an individual family with DC. Exceptions are the A353V mutation in DKC1 and R282H and R282C TINF2 that are recurrent mutations. Occasionally DKC1 or TERT gene mutations have been described independently in more than one family. Interestingly the recurrent A353V mutation in DKC1 and the R282H and R282C in TINF2 most frequently occur sporadically (see also above) suggesting that these occur within mutational hotspots and indeed these three mutations all arise from the relatively frequent CpG to TpG transitions that can occur by deamination of the cytosine residue [37]. This cannot wholly explain the high frequencies of these mutations, and it is expected the role of these frequently mutated residues will hold important clues about the pathogenetic mechanism.
The fact that many of the gene alterations are only described once in a patient or family makes it difficult to determine whether the identified sequence alteration is indeed responsible for disease (pathogenic). This is more difficult when the same gene alteration has also been identified in unaffected family members or even in unrelated individuals, as can happen because of the variable penetrance of TERC and TERT mutations. Functional in vitro assays of telomerase activity or quantitative measurements of the level of telomerase RNA may support the diagnosis but often lead to variable results depending on the investigator. Moreover in vitro telomerase activity may not necessarily reflect the telomerase activity at the telomere end in vivo. Family studies and segregation with disease or short telomeres may lend support, but cannot on their own necessarily consolidate or exclude the pathogenicity of a novel identified gene alteration.
Several polymorphism have been described in DKC1, TERC, TERT and TINF2 genes, some of which have been shown to affect the expression levels of the respective cDNA or decrease the in vitro telomerase activity (functional polymorphism). However, to what extent these functional polymorphisms contribute or predispose to the development of disease is unclear and remains to be determined. In the context of genetic counseling or clinical decision-making such genetic variations should be carefully interpreted to avoid unnecessary anxiety in a patient who may remain healthy or to exclude a transplant from a sibling donor who otherwise has no clinical or laboratory signs of disease.
The role of telomere length measurements in the diagnosis of DC
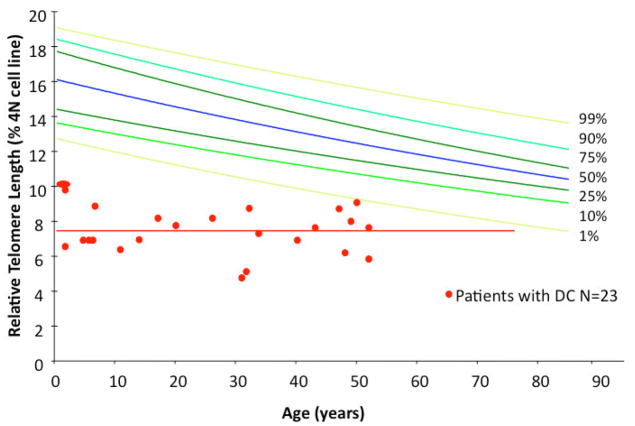
That all of the known DC-associated genes are involved in telomere maintenance suggests that telomere dysfunction is the common denominator in this disease and that dysfunctional telomeres play an important role in pathogenesis. Indeed, patients with DC and BMF have very short telomeres (see Fig. 2) [50,67,68]. The measurement of telomere length in peripheral blood cells has therefore become increasingly used as a screening test to identify patients with DC. Amongst patients with BMF the finding of very short telomeres (≪ 1st percentile in peripheral mononuclear cells or lymphocytes) has been shown to be highly sensitive, although not necessarily specific, for the diagnosis of DC. In other words, normal telomere length excludes DC as a cause of BMF, whereas the finding of short telomeres is not necessarily diagnostic of DC [50]. Other BMF syndromes have also been shown to be associated with short telomeres but the telomeres are usually not as short as in patients with BMF due to DC, particularly in children. Leukocyte telomere length in normal healthy individuals decreases with age, as seen when age is plotted against telomere length for a large number of individuals (see Fig. 2). Interestingly, this is not seen when the same data from patients with DC and BMF is plotted; rather, patients with DC have very short telomeres whose length does not vary with age. This suggests that at the time of clinically apparent BMF peripheral blood leukocytes have reached their minimal length in patients with DC [50]. Consequently, in children with BMF the difference in telomere lengths between patients with and without DC is dramatic, whereas in older patients with BMF the telomere lengths of patients with and without DC overlap. Thus, telomere length measurements are a more sensitive diagnostic tool for detecting DC in the setting of BMF in children than in older adults [50]. Mutation carriers (mainly TERC and TERT) who do not have clinical manifestations of BMF often have telomere lengths that are shorter than average, but due to the overlapping distribution of telomere lengths in the population this is not a sensitive test with which to identify a mutation carrier from a normal control. This is in particularly true in families with DC, because short telomeres, but not the pathogenic mutation, may have been inherited from the affected parent. The clinical significance of short telomeres by inheritance in the absence of a DC gene mutation is currently under study. The investigation of large families has shown that two generations without a DC gene mutation are needed to restore normal telomere length in these families [62]. Similarly, these family studies have shown that in the absence of BMF, normal telomere length does not exclude a silent mutation carrier. Thus, in the absence of BMF, genetic testing is essential for the diagnosis of DC or for the identification of a silent mutation carrier [50].
Figure 2.
Telomere length distribution in normal controls and patients with BMF due to DC. The colored lines represent the 1st, 5th, 25th, 50th, 75th, 95th and 99th percentiles of telomere length determined in peripheral blood mononuclear cells in 250 normal controls between the ages of 1 and 94 years old. In red are the telomere lengths in peripheral blood mononuclear cells isolated from patients with BMF and DC in whom a mutation in a DC associated gene was confirmed by genetic testing.
Model for the pathogenesis of BMF and malignancy in patients with DC
Telomere ends are protected from degradation and inappropriate recombination mainly by two mechanisms: 1) the formation of a loop like structure (t-loop), and 2) the shelterin complex (see Fig. 1). Dysfunctional telomeres were initially thought to be telomeres that have become too short to adopt the t-loop structure. Recently it has been shown that telomeres may also become dysfunctional due to a capping defect caused by abnormalities in the shelterin complex that may be independent of telomere length [69,70]. Our working model is that hematopoietic progenitor cells that have dysfunctional telomere ends will trigger a DNA damage response that will lead them to irreversibly cease to divide and undergo senescence or possibly die. Dysfunctional telomeres are most likely to occur first in rapidly dividing hematopoietic progenitor cells. The loss of dividing progenitor cells starts a vicious cycle by increasing the number of stem cells recruited into cell cycle until the telomeres of hematopoietic stem cells are eroded. The loss of dividing hematopoietic progenitor cells, and finally stem cells, leads to irreversible BMF. Dysfunctional telomeres activate the p53 pathway and increase genomic instability leading eventually to a cell crisis, cell cycle arrest, senescence or cell death. Rare cells that escape cell senescence or cell death and have acquired a mechanism to maintain telomere integrity emerge from the crisis as potentially malignant cells. A schematic diagram of our model of BMF and tumorigenesis is shown in Fig. 3.
In normal individuals the telomeres are maintained by three mechanisms: 1) hematopoietic stem cells have low level telomerase activity that compensates, at least in part, for the gradual loss of telomere length caused by cell division, maintains telomere integrity, and protects from DNA damage; 2) under normal circumstances most stem cells are quiescent; 3) telomeres in normal individuals are long enough that even as telomeres shorten with age they rarely reach the critical length that leads to irreversible cell cycle arrest. In patients with DC four mechanisms may directly, or indirectly through increased p53 activation and increased stem cell turnover (replicative stress) contribute to dysfunctional telomeres. These include: 1) reduced telomerase activity at the telomere end, 2) the inheritance of short telomeres; 3) increased DNA damage response at the end of telomeres; 4) oxidative DNA damage in the environment of BMF; and 5) for the X-linked and rare autosomal recessive forms of DC impaired ribosome biogenesis (see Fig. 3).
Age-related variable expression of mutations in telomere maintenance genes
Abnormalities caused by mutations in telomere maintenance genes vary with the age of onset of disease (see also Fig. 4). Onset at a young age is consistent with severe disease and often causes death in early childhood. The clinical picture is characterized by the presence of immunodeficiency, BMF, microcephaly, cerebellar hypoplasia, and growth retardation. Crohn-like gut abnormalities and malabsorption often additionally complicate early onset of disease. This set of abnormalities, originally described as Hoyeraal Hreidarrson syndrome (HSS), is now recognized to be an early onset and severe form of DC (associated with mutations in DKC1, TINF2 and other unknown genes). Sometimes BMF is found with bilateral retinopathy and some other DC features in infants in Revesz syndrome, caused by mutations in TINF2.
In older children and teenagers, classic DC with the triad of mucocutaneous features and BMF is the most frequent DC presentation and is caused by mutations in DKC1 or TINF2, with TINF2 patients presenting on average earlier than DKC1 patients. Occasionally the classic presentation of DC is caused by homozygous or compound heterozygous mutations in TERC or TERT [41,53,54].
Later in life TERC and TERT gene mutations are often found in patients presenting with aplastic anemia in the absence of mucocutaneous features. Older patients may also present with familial or apparently idiopathic PF or with liver disease. Mutations in TERC or TERT are usually responsible for these cases.
Cancer in patients with DC usually occurs in the third decade with head and neck cancer and MDS being the most common malignancies and in the early fifties with MDS/AML being more frequent. Mainly patients with moderate or mild forms of DC or those who have received hematopoietic stem cell transplant develop malignancies, whereas patients with severe forms of DC usually die from the disease before the development of malignancy.
The difference in age dependent disease expression is intriguing and unique. It suggests that depending on the degree of telomere maintenance dysfunction, different tissues are predominantly affected. This might be due to the fact that different highly proliferative tissues “run out” of telomeres at different time points. For example cerebellar neuronal cells might run out of telomeres only in very severe cases of telomere dysfunction, whereas hematopoietic progenitor cells are much more sensitive and might be affected by only minimal changes. An alternative explanation is that it is not telomere dysfunction but the pattern and levels of p53 expression and the sensitivity of cells to increased levels of p53 that dictates the clinical picture. For example high levels of p53 expression in embryonic development causes cell death in neuronal cells and retina, moderate levels of p53 expression causes replicative senescence in hematopoietic progenitor cells, whereas low but chronic level of p53 expression causes senescence in pneumocytes and fibrosis in lung and liver tissue [71].
Summary
Insights gained into telomere biology have transformed our understanding of DC and the approach to patients and families with this disorder. In turn studying the clinical manifestations of DC have shed light on the fundamental importance of maintaining the telomere ends. A better understanding of how telomeres are maintained may eventually yield new treatment options for patients and families with DC.
Acknowledgments
We are grateful to members of our laboratories, past and present for interesting discussions about DC. Work in the authors laboratories was supported by NIH/NCI grants R01 CA105312 (MB) and R01 CA106995 (PM). We apologize to the authors of original work we were not able to cite due to the limited numbers of references permitted.
Abbreviations
- AML
Acute myeloid leukemia
- BMF
Bone marrow failure
- DC
Dyskeratosis congenita
- HHS
Hoyeraal Hreidarrson syndrome
- MDS
Myelodysplastic Syndrome
- PF
Pulmonary fibrosis
- POT1
protection of telomeres 1
- TIN2
TRF1-interacting nuclear factor
- TRF1
telomeric repeat binding factor 1
- TRF2
telomeric repeat binding factor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bessler M, Mason P, Link D, Wilson D. Nathan and Oski’s Hematology of Infancy and Childhood. In: Orkin S, Nathan D, Ginsburg D, Look A, Fisher D, Lux S, editors. Saunders; Philadelphia: 2008. pp. 307–395. [Google Scholar]
- 2.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–52. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–81. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE. Structure and variability of human chromosome ends. Mol Cell Biol. 1990;10:518–27. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–73. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 6.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85:6622–6. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 8.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–9. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 10.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–98. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 11.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–9. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–3. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 13.Fu D, Collins K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol Cell. 2007;28:773–85. doi: 10.1016/j.molcel.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, Kim SK, Artandi SE. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 16.Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677–85. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- 17.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–8. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 18.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–8. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–67. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–82. [PMC free article] [PubMed] [Google Scholar]
- 21.Harley CB. Telomerase therapeutics for degenerative diseases. Curr Mol Med. 2005;5:205–11. doi: 10.2174/1566524053586671. [DOI] [PubMed] [Google Scholar]
- 22.Cole H, Rauschkolb J, Toomey J. Dyskeratosis congenita with pigmentation, dystrophia unguis and leukokeratosis oris. Archives of Dermatology and Syphyligraphie. 1930;21:71–95. doi: 10.1001/archderm.1955.01540280027005. [DOI] [PubMed] [Google Scholar]
- 23.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–79. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 24.Knight S, Vulliamy T, Copplestone A, Gluckman E, Mason P, Dokal I. Dyskeratosis Congenita (DC) Registry: identification of new features of DC. Br J Haematol. 1998;103:990–6. doi: 10.1046/j.1365-2141.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 25.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–57. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baykal C, Kavak A, Gulcan P, Buyukbabani N. Dyskeratosis congenita associated with three malignancies. J Eur Acad Dermatol Venereol. 2003;17:216–8. doi: 10.1046/j.1468-3083.2003.00585.x. [DOI] [PubMed] [Google Scholar]
- 27.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–8. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–5. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999;19:567–76. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–5. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–24. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 32.Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, Al-Qurashi FH, Aljurf M, Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–29. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci U S A. 2008;105:8073–8. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason PJ, Wilson DB, Bessler M. Dyskeratosis congenita -- a disease of dysfunctional telomere maintenance. Curr Mol Med. 2005;5:159–70. doi: 10.2174/1566524053586581. [DOI] [PubMed] [Google Scholar]
- 35.Vulliamy TJ, Dokal I. Dyskeratosis congenita: the diverse clinical presentation of mutations in the telomerase complex. Biochimie. 2008;90:122–30. doi: 10.1016/j.biochi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–9. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104:7552–7. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 40.Calado RT, Regal JA, Kleiner DE, Schrump DS, Peterson NR, Pons V, Chanock SJ, Lansdorp PM, Young NS. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS One. 2009;4:e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du HY, Pumbo E, Manley P, Field JJ, Bayliss SJ, Wilson DB, Mason PJ, Bessler M. Complex inheritance pattern of dyskeratosis congenita in two families with 2 different mutations in the telomerase reverse transcriptase gene. Blood. 2008;111:1128–30. doi: 10.1182/blood-2007-10-120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirwan M, Vulliamy T, Marrone A, Walne AJ, Beswick R, Hillmen P, Kelly R, Stewart A, Bowen D, Schonland SO, Whittle AM, McVerry A, Gilleece M, Dokal I. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia. Hum Mutat. 2009;30:1567–73. doi: 10.1002/humu.21115. [DOI] [PubMed] [Google Scholar]
- 43.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hills M, Lansdorp PM. Short telomeres resulting from heritable mutations in the telomerase reverse transcriptase gene predispose for a variety of malignancies. Ann N Y Acad Sci. 2009;1176:178–90. doi: 10.1111/j.1749-6632.2009.04565.x. [DOI] [PubMed] [Google Scholar]
- 45.Yu CE, Oshima J, Fu YH, Wijsman EM, Hisama F, Alisch R, Matthews S, Nakura J, Miki T, Ouais S, Martin GM, Mulligan J, Schellenberg GD. Positional cloning of the Werner’s syndrome gene. Science. 1996;272:258–62. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- 46.O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 47.McNees CJ, Tejera AM, Martinez P, Murga M, Mulero F, Fernandez-Capetillo O, Blasco MA. ATR suppresses telomere fragility and recombination but is dispensable for elongation of short telomeres by telomerase. J Cell Biol. 2010;188:639–52. doi: 10.1083/jcb.200908136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pennarun G, Hoffschir F, Revaud D, Granotier C, Gauthier LR, Mailliet P, Biard DS, Boussin FD. ATR contributes to telomere maintenance in human cells. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkp1248. Epub ahead of print Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359:2168–70. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 50.Du HY, Pumbo E, Ivanovich J, An P, Maziarz RT, Reiss UM, Chirnomas D, Shimamura A, Vlachos A, Lipton JM, Goyal RK, Goldman F, Wilson DB, Mason PJ, Bessler M. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113:309–16. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–5. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 52.Marrone A, Sokhal P, Walne A, Beswick R, Kirwan M, Killick S, Williams M, Marsh J, Vulliamy T, Dokal I. Functional characterization of novel telomerase RNA (TERC) mutations in patients with diverse clinical and pathological presentations. Haematologica. 2007;92:1013–20. doi: 10.3324/haematol.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marrone A, Walne A, Tamary H, Masunari Y, Kirwan M, Beswick R, Vulliamy T, Dokal I. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood. 2007;110:4198–205. doi: 10.1182/blood-2006-12-062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ly H, Schertzer M, Jastaniah W, Davis J, Yong SL, Ouyang Q, Blackburn EH, Parslow TG, Lansdorp PM. Identification and functional characterization of 2 variant alleles of the telomerase RNA template gene (TERC) in a patient with dyskeratosis congenita. Blood. 2005;106:1246–52. doi: 10.1182/blood-2005-01-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batra R, Charizanis K, Swanson MS. Partners in Crime: Bidirectional Transcription in Unstable Microsatellite Disease. Hum Mol Genet. 2010 doi: 10.1093/hmg/ddq132. Epub ahead of print Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker FO. Huntington’s Disease. Semin Neurol. 2007;27:143–50. doi: 10.1055/s-2007-971176. [DOI] [PubMed] [Google Scholar]
- 57.Sutherland GR, Richards RI. Simple tandem DNA repeats and human genetic disease. Proc Natl Acad Sci U S A. 1995;92:3636–41. doi: 10.1073/pnas.92.9.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 59.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 60.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36:447–9. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 61.Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–4. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldman F, Bouarich R, Kulkarni S, Freeman S, Du HY, Harrington L, Mason PJ, Londono-Vallejo A, Bessler M. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci U S A. 2005;102:17119–24. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rashid R, Liang B, Baker DL, Youssef OA, He Y, Phipps K, Terns RM, Terns MP, Li H. Crystal structure of a Cbf5-Nop10-Gar1 complex and implications in RNA-guided pseudouridylation and dyskeratosis congenita. Mol Cell. 2006;21:249–60. doi: 10.1016/j.molcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 64.Li L, Ye K. Crystal structure of an H/ACA box ribonucleoprotein particle. Nature. 2006;443:302–7. doi: 10.1038/nature05151. [DOI] [PubMed] [Google Scholar]
- 65.Liang B, Zhou J, Kahen E, Terns RM, Terns MP, Li H. Structure of a functional ribonucleoprotein pseudouridine synthase bound to a substrate RNA. Nat Struct Mol Biol. 2009;16:740–6. doi: 10.1038/nsmb.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du HY, Idol R, Robledo S, Ivanovich J, An P, Londono-Vallejo A, Wilson DB, Mason PJ, Bessler M. Telomerase reverse transcriptase haploinsufficiency and telomere length in individuals with 5p- syndrome. Aging Cell. 2007;6:689–97. doi: 10.1111/j.1474-9726.2007.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001;27:353–7. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 68.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–47. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 70.Gu BW, Bessler M, Mason PJ. A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proc Natl Acad Sci U S A. 2008;105:10173–8. doi: 10.1073/pnas.0803559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marine J-C, Francoz S, Maetens M, Wahl G, Toledo F, Lozano G. Keeping p53 in check: essential and synergistic functions of MDM2 and MDM4. Cell Death Diff. 2006;13:927–34. doi: 10.1038/sj.cdd.4401912. [DOI] [PubMed] [Google Scholar]