Abstract
Background: Low dietary choline intake has been proposed to increase the risk of neural tube defects (NTDs) in human populations. Mice with reduced Shmt1 expression exhibit a higher frequency of NTDs when placed on a folate- and choline-deficient diet and may represent a model of human NTDs. The individual contribution of dietary folate and choline deficiency to NTD incidence in this mouse model is not known.
Objective: To dissociate the effects of dietary folate and choline deficiency on Shmt1-related NTD sensitivity, we determined NTD incidence in embryos from Shmt1-null dams fed diets deficient in either folate or choline.
Design: Shmt1+/+ and Shmt1−/− dams were maintained on a standard AIN93G diet (Dyets), an AIN93G diet lacking folate (FD), or an AIN93G diet lacking choline (CD). Virgin Shmt1+/+ and Shmt1−/− dams were crossed with Shmt1+/− males, and embryos were examined for the presence of NTDs at embryonic day (E) 11.5 or E12.5.
Results: Exencephaly was observed only in Shmt1−/− embryos isolated from dams maintained on the FD diet (P = 0.004). Approximately 33% of Shmt1−/−embryos (n = 18) isolated from dams maintained on the FD diet exhibited exencephaly. NTDs were not observed in any embryos isolated from dams maintained on the CD (n = 100) or control (n = 152) diets or in any Shmt1+/+ (n = 78) or Shmt1+/− embryos (n = 182).
Conclusion: Maternal folate deficiency alone is sufficient to induce NTDs in response to embryonic Shmt1 disruption.
See corresponding editorial on page 1.
INTRODUCTION
NTDs6 represent common congenital abnormalities arising from the failure of neural tube closure. The most prevalent and severe of these disorders include anencephaly and spina bifida, which result from a failure of rostral and caudal neural tube closure, respectively. Collectively, NTDs affect ∼1 in 1000 births worldwide (1). Analyses of human inheritance patterns have shown that NTD susceptibility arises largely from deleterious gene-nutrient interactions. Among these, low maternal folate status (2), in conjunction with several folate-related gene variants (3, 4), confers the highest risk of NTD occurrence. However, the underlying mechanism or mechanisms by which folate and folate-gene interactions affect NTD risk remain unknown.
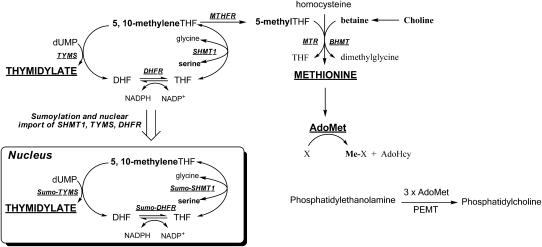
In the cell, folates act as essential cofactors for the de novo synthesis of purine and thymidine nucleotides and for the remethylation of homocysteine to methionine (Figure 1; reviewed in reference 5). Elevated homocysteine is cytotoxic (6), and methionine is required for the synthesis of AdoMet, the key methyl donor for cellular methylation reactions (Figure 1). Thus, folate can affect NTD risk by 1) impairing nucleotide biosynthesis, 2) elevating homocysteine, or 3) altering cellular methylation potential. Debate persists as to which mechanism is the most relevant for NTD outcomes.
FIGURE 1.
Nuclear and cytoplasmic folate-mediated one-carbon metabolism. The one-carbon unit and products of one-carbon metabolism are shown in bold. The remethylation of homocysteine to methionine can occur either through the folate-dependent pathway involving 5-methylTHF as the methyl donor or through a folate-independent pathway involving betaine as the methyl donor. The PEMT-catalyzed formation of phosphatidylcholine is an example of an AdoMet-dependent methylation reaction. The de novo thymidylate biosynthesis pathway translocates to the nucleus during the S phase. AdoHcy, S-adenosylhomocysteine; AdoMet, S-adenosylmethionine; BHMT, betaine:homocysteine methyltransferase; DHF, dihydrofolate; DHFR, dihydrofolate reductase; dUMP, deoxyuridine monophosphate; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; PEMT, phosphatidylethanolamine methyltransferase; SHMT1, serine hydroxymethyltransferase 1; THF, tetrahydrofolate; TYMS, thymidylate synthase.
Interestingly, recent epidemiologic evidence suggests that maternal choline intake also influences NTD risk (7, 8). Choline is an essential nutrient that can be obtained from the diet or synthesized de novo from 3 molecules of AdoMet (Figure 1). Choline is required for the synthesis of membrane phospholipids and for the neurotransmitter acetylcholine (9) and can be degraded for the remethylation of homocysteine to methionine. The intersection of folate and choline metabolism at the homocysteine methylation cycle suggests that the increased NTD risk associated with deficiency of either of these nutrients may reflect a common etiology related to impaired cellular methylation, homocysteine accumulation, or both (10). However, some animal (11, 12) and epidemiologic (7, 8) studies have suggested that low choline status may influence NTD risk independently of folate status.
Previous work in our laboratory has shown that embryonic Shmt1 disruption leads to an increased incidence of exencephaly when dams are maintained on an FCD diet (13). SHMT1 is 1 of 3 enzymes within the folate metabolic pathway that is involved in de novo thymidylate biosynthesis (Figure 1). Therefore, increased occurrence of NTDs in Shmt1-null animals suggests the involvement of decreased nucleotide biosynthesis in folate-related NTD pathogenesis. However, because the FCD diet also resulted in increased maternal homocysteine, we cannot disregard the contribution of dietary choline deficiency to NTD pathology in this mouse model. To dissociate the relative contributions of dietary folate and choline deficiency to Shmt1-related NTD sensitivity, we assessed NTD incidence in Shmt1-null embryos from dams fed diets deficient in either folate or choline.
MATERIALS AND METHODS
Experimental animals and diets
Shmt-null mice are viable and fertile, and their generation has been described previously (14). Shmt1+/−mice were maintained as a heterozygous breeding colony on a 129SvEv background (Taconic Farms) under specific-pathogen-free conditions. All animal experiments were approved by the Cornell Institute Animal Care and Use Committee according to the guidelines of the Animal Welfare Act and all applicable state and federal laws. Animals were housed under a 12 h light-dark cycle in a temperature- and humidity-controlled room and received ad libitum access to food and water. At weaning, Shmt1+/+ and Shmt1−/− dams were randomly assigned to a standard AIN93G (control) diet (Dyets), an FD diet, or a CD diet and were maintained on this diet throughout breeding and gestation until killed.
Breeding and embryonic assessment
Virgin Shmt1+/+ and Shmt1−/− dams 42–120 d old were housed overnight with Shmt1+/− males as described previously (13). The following morning, dams were examined for the presence of a vaginal plug. The morning of the plug was designated as E0.5. Dams were killed by carbon dioxide asphyxiation at E11.5–12.5. Gravid uteri were removed, and all implants and resorption sites were recorded. Embryos were examined for the presence of NTDs under a dissecting scope (Leica) and CR length was measured. Although on initial examination and scoring of embryos, investigators were not blinded as to maternal genotype and diet, a subset of embryos was rescored by a blinded investigator to verify accurate scoring. Embryos that were damaged during extraction, that exhibited NTDs, or that were undergoing resorption or dying were not included in the analysis of CR length. Resorption rate was calculated as the number of resorptions per litter divided by the number of implants. Embryos in the process of resorption were not included in the determination of the number of implants. Yolk sacs were removed and stored at −20°C for genotype analysis. Livers were removed from dams and flash-frozen in liquid nitrogen; blood was obtained through cardiac puncture and collected into heparin-coated tubes. Plasma was isolated via centrifugation at 8000 rpm for 5 min and then flash-frozen in liquid nitrogen.
Genotype analysis
Genotyping for the Shmt1+/− allele (14) and sex (15) followed established protocols.
Determination of metabolites
The effects of Shmt1 disruption and dietary folate or choline deficiency on folate status and markers of choline metabolism were determined in plasma isolated from dams maintained on the respective diets from weaning through gestation. Folate concentrations were quantified by using a microbiological assay as previously described (16). Liquid chromatography–mass spectrometry was used to measure choline, betaine, and dimethylglycine (17), as well as phosphatidylcholine, lysophosphatidylcholine, glycerophosphocholine, phosphocholine, and sphingomyelin (18).
Measurement of maternal genomic uracil
Uracil content in hepatic maternal nuclear DNA was determined by gas chromatography–mass spectrometry as previously described (14), with the following modifications: 1) the RNase incubation was removed, 2) 1.0–2.0 μg DNA was used, and 3) analysis of uracil-3,5-bis(trifluoromethyl)benzyl bromide was carried out on a Shimadzu QP2010Plus (Shimadzu).
Statistical analysis
Analysis of NTD incidence was conducted by using Fisher's exact test for independence with Bonferroni's correction (n=3). The following comparisons of NTD incidence were made: 1) Shmt1−/− embryos from dams fed an FD diet compared with from dams fed a control diet, 2) Shmt1−/− embryos from dams fed an FD diet compared with those from dams fed a CD diet, and 3) Shmt1−/− compared with Shmt1+/+ embryos from dams fed an FD diet. Analyses of embryonic CR length, total litter resorptions, and implants were performed by 2-factor ANOVA with Tukey's honestly significant difference for post hoc analysis. Litter was incorporated in the model as a random effect. Independent variables included maternal genotype and diet. For CR length, embryonic age and sex were also included as independent variables. To dissociate the effect of maternal Shmt1 genotype from embryonic genotype on CR length, analysis was also performed on Shmt1+/− embryos alone. Resorption rate was calculated as the ratio of resorptions/total implants per litter, and square-root transformation was applied to normalize the data. Analyses of uracil concentrations and metabolites were conducted by 2-factor ANOVA with Tukey's honestly significant difference for post hoc analysis. Independent variables included maternal genotype and diet. Log transformation was applied to the analysis of glycerophosphocholine concentrations to normalize the data. Fisher's exact tests were performed by using StatXact (Cytel Inc) . All other tests were performed in JMP, version 8.02 (SAS Institute).
RESULTS
Dietary folate, but not choline deficiency, sensitized SHMT1-deficient embryos to exencephaly
To dissociate the contribution of impaired folate and choline status to NTD risk in SHMT1-deficient embryos, the incidence of NTDs was examined in embryos isolated from crosses of Shmt1+/− males to Shmt1+/+ and Shmt1−/− dams maintained on either a control, an FD, or a CD diet from weaning through gestation. Shmt1+/− dams were not included in these crosses to most effectively compare the effect of maternal diet on NTD risk in Shmt1+/+ and Shmt1−/− embryos, because we have previously shown that Shmt1−/− embryos are at a significantly higher risk than Shmt1+/− embryos (13). There is also no effect of maternal genotype in this model (13).
Exencephaly was observed only in embryos isolated from dams maintained on the FD diet (P = 0.004; Table 1; Figure 2). All exencephalic embryos isolated were also Shmt1−/− (Table 1). Approximately 7% of all Shmt1−/−embryos and 33% of Shmt1−/−embryos isolated from dams maintained on the FD diet exhibited exencephaly. Importantly, NTDs were not observed in any litters isolated from dams maintained on the CD or control diets or in any embryos that were Shmt1+/+ or Shmt1.+/−
TABLE 1.
Frequency of NTDs in Shmt1−/− embryos as a function of maternal diet1
| Embryonic genotype |
|||
| Maternal diet | Shmt1+/+ | Shmt1+/− | Shmt1−/− |
| AIN93G | |||
| No. of embryos | 32 | 78 | 42 |
| No. of NTDs | 0 | 0 | 0 |
| AIN93G minus choline | |||
| No. of embryos | 19 | 53 | 28 |
| No. of NTDs | 0 | 0 | 0 |
| AIN93G minus folate | |||
| No. of embryos | 27 | 51 | 18 |
| No. of NTDs | 0 | 0 | 6 (33%)2 |
Differences between groups were analyzed with Fisher's exact test of independence with Bonferroni's correction (n = 3). All NTDs represent exencephaly and were assessed between gestational days 11.5–12.5. NTDs, neural tube defects.
P = 0.0044 for the number of NTDs in Shmt1−/− embryos isolated from dams fed the AIN93G diet (Dyets) lacking folate compared with Shmt1−/− embryos isolated from dams fed the control AIN93G diet; P = 0.020 for the number of NTDs in Shmt1−/− embryos isolated from dams fed the AIN93G diet lacking folate compared with Shmt1−/− embryos isolated from dams fed the AIN93G diet lacking choline; P < 0.001 for the number of NTDs in Shmt1−/− embryos compared with Shmt1+/+ embryos isolated from dams fed the AIN93G diet lacking folate.
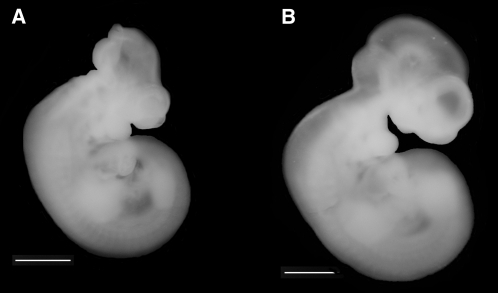
FIGURE 2.
A: Shmt1−/− embryos isolated from dams fed a folate-deficient diet exhibit exencephaly. B: No neural tube defects were observed in Shmt1+/− littermates.
Maternal diet and maternal genotype significantly interacted to affect embryonic growth as determined by measurement of CR length at E11.5–12.5 (Table 2). Embryos isolated from Shmt1−/− dams fed the FD diet exhibited the shortest CR length across all groups, and embryos from Shmt1−/− dams were shorter than those from Shmt1+/+ dams fed the control diet (F = 7.037, P = 0.0018). Female embryos were also significantly shorter than males (F = 7.22, P = 0.0078; data not shown). Because the effect of maternal genotype was confounded by the distribution of embryonic genotypes, we determined the effect of maternal genotype for Shmt1+/− embryos alone to confirm a true effect of maternal genotype on CR length. The interaction of maternal genotype and diet (F = 5.037, P = 0.0099) remained significant when only Shmt1+/− embryos were used for analysis.
TABLE 2.
Average embryonic CR length, average number of implants per litter, and average resorption rate per litter as a function of maternal Shmt1 genotype and diet1
| Dam genotype | CR length | Implants | Resorption rate |
| mm | n | ||
| AIN93G | |||
| Shmt1+/+ | 8.08 ± 0.3 | 6.03 ± 0.8 | 0.08 ± 0.1 |
| Shmt1−/− | 7.09 ± 0.3 | 5.93 ± 0.6 | 0.01 ± 0.1 |
| AIN93G minus choline | |||
| Shmt1+/+ | 6.60 ± 0.3 | 5.15 ± 0.9 | 0.10 ± 0.1 |
| Shmt1−/− | 7.48 ± 0.4 | 6.06 ± 0.8 | 0.21 ± 0.1 |
| AIN93G minus folate | |||
| Shmt1+/+ | 7.38 ± 0.4 | 4.72 ± 0.8 | 0.61 ± 0.1 |
| Shmt1−/− | 6.38 ± 0.4 | 4.89 ± 0.8 | 0.17 ± 0.1 |
| P value | |||
| Diet effect | 0.04262 | NS | 0.00233 |
| Genotype effect | NS | NS | NS |
| Diet × genotype effect | 0.00184 | NS | NS |
All values are means ± SEMs. n = 30–65 embryos for CR length; n = 8–14 litters for number of implants and resorption rate. Differences between genotypes, diets, and the interaction of genotype × diet were analyzed by 2-factor ANOVA by using Tukey's honestly significant difference for post hoc analysis. CR, crown-rump.
Post hoc analysis did not show any significant comparisons.
The AIN93G (Dyets) folate-deficient diet was significantly different from control.
Embryos isolated from Shmt1+/+ dams fed a control diet were significantly different from embryos isolated from Shmt1−/− dams fed a folate-deficient diet and from Shmt1+/+ dams fed a choline-deficient diet.
Total implants and resorption rates were examined to determine the effects of dietary folate and choline deficiency and maternal Shmt1 disruption on fertility. The FD diet was associated with a significant increase in resorption rate (F = 6.749, P = 0.0023; Table 2), as previously observed for the FCD diet (13), but Shmt1 genotype did not significantly influence the resorption rate. The CD diet did not affect resorption rates. No significant effect of diet or maternal Shmt1 genotype or any interaction of these factors was observed on the number of implants per litter (Table 2).
Maternal Shmt1 disruption altered total folate concentrations but not markers of choline metabolism
To investigate the metabolic alterations associated with NTD pathogenesis, plasma folate status was determined in pregnant Shmt1+/+and Shmt1−/− dams fed the control, CD, and FD diets. Plasma folate concentrations were significantly reduced in response to maternal Shmt1 disruption (F = 5.806, P = 0.019; Table 3) and dietary folate deficiency (F= 37.44, P < 0.0001; Table 3). Across all 3 diets, Shmt1−/− animals showed an approximate 25% reduction in total folate concentrations as compared with Shmt1+/+mice (Table 3). The FD diet lowered total folate concentrations by ∼80% as compared with the control and CD diets (Table 3). The effect of Shmt1 disruption on plasma folate concentrations was greatest with the FD diet (>55% reduction), although the interaction of Shmt1 genotype and diet was not significant. The CD diet did not affect plasma folate concentrations.
TABLE 3.
Metabolic profile of plasma from Shmt1+/+ and Shmt1−/− dams maintained on control, CD, and FD diets1
| Diet |
P value |
||||||||
| AIN93G |
AIN93G minus choline |
AIN93G minus folate |
|||||||
| Metabolite | Shmt1+/+ | Shmt1−/− | Shmt1+/+ | Shmt1−/− | Shmt1+/+ | Shmt1−/− | Diet effect | Genotype effect | Diet × genotype effect |
| Folate (ng/mL) | 40.00 ± 3.9 | 36.34 ± 3.9 | 43.83 ± 4.4 | 29.80 ± 3.7 | 10.60 ± 3.9 | 4.47 ± 4.1 | <0.00012 | 0.019 | NS |
| Choline (nmol/mL) | 28.84 ± 2.5 | 31.92 ± 2.5 | 30.69 ± 2.8 | 26.78 ± 2.4 | 30.45 ± 2.5 | 29.80 ± 2.6 | NS | NS | NS |
| Betaine (nmol/mL) | 35.75 ± 2.5 | 39.10 ± 2.5 | 26.79 ± 2.8 | 25.40 ± 2.4 | 33.47 ± 2.5 | 32.06 ± 2.6 | 0.00023 | NS | NS |
| Dimethylglycine (nmol/mL) | 5.25 ± 0.4 | 6.10 ± 0.4 | 3.66 ± 0.5 | 3.66 ± 0.4 | 5.57 ± 0.4 | 5.37 ± 0.4 | <0.00013 | NS | NS |
| Phosphatidylcholine (nmol/mL) | 890.10 ± 89.9 | 838.19 ± 85.3 | 748.30 ± 102.0 | 708.32 ± 81.4 | 892.30 ± 85.3 | 832.03 ± 89.9 | NS | NS | NS |
| Lysophosphtidylcholine (nmol/mL) | 548.91 ± 58.9 | 546.65 ± 55.9 | 605.75 ± 66.8 | 612.52 ± 53.3 | 610.94 ± 55.9 | 650.75 ± 58.9 | NS | NS | NS |
| Glycerophosphocholine (nmol/mL) | 89.86 ± 16.5 | 73.73 ± 16.5 | 131.46 ± 19.7 | 93.4 ± 15.7 | 55.64 ± 16.5 | 91.95 ± 17.4 | NS | NS | 0.0254 |
| Phosphocholine (nmol/mL) | 5.07 ± 0.9 | 6.96 ± 0.9 | 4.59 ± 1.1 | 4.74 ± 0.8 | 6.67 ± 1.0 | 3.93 ± 0.9 | NS | NS | 0.0545 |
| Sphingomyelin (nmol/mL) | 87.16 ± 14.6 | 90.60 ± 13.9 | 103.50 ± 16.6 | 86.33 ± 13.2 | 111.88 ± 13.9 | 103.64 ± 14.6 | NS | NS | NS |
All values are means ± SEMs. n = 7–11 per group. Differences between genotypes, diets, and genotype × diet effects were analyzed by 2-factor ANOVA by using Tukey's honestly significant difference post hoc analysis. CD, AIN93G diet (Dyets) deficient in choline; FD, AIN93G diet deficient in folate.
The FD diet was significantly different from the control and CD diets.
The CD diet was significantly different from the control and FD diets.
Shmt1+/+ dams fed an FD diet were different from Shmt1+/+ and Shmt1−/− dams fed a CD diet.
Post hoc analysis did not yield significant differences.
Alterations in choline metabolism were determined by measuring plasma choline metabolites in pregnant dams. Dietary choline deficiency significantly lowered betaine and dimethylglycine concentrations by >20% and >35%, respectively, as compared with the control and FD diets (F = 10.35, P = 0.0002 for betaine; F = 14.28, P < 0.0001 for dimethylglycine; Table 3). In addition, a significant interaction of Shmt1 genotype and diet (F = 3.977, P = 0.0248; Table 3) was observed for concentrations of glycerophosphocholine. Post hoc analysis showed that dietary folate deficiency in the Shmt1+/+ dams significantly lowered glycerophosphocholine concentrations in comparison with the Shmt1+/+and Shmt1−/− dams fed a CD diet (F = 3.977, P = 0.0248; Table 3). Although no other choline metabolites were significantly affected by dietary folate deficiency, there was a trend toward a significant interaction of Shmt1 genotype and diet on concentrations of phosphocholine (F = 3.102, P = 0.054; Table 3); however, none of the individual comparisons were significant.
Maternal hepatic uracil content was not altered in response to Shmt1 disruption or diet
To determine the contribution of impaired nucleotide biosynthesis to NTD risk in the SHMT1-deficient mouse model, uracil content was measured in nuclear DNA isolated from livers of Shmt1+/+ and Shmt1−/− dams maintained on the control, CD, or FD diets. There was no effect of Shmt1 genotype on liver uracil concentrations (Table 4). Uracil concentration were similarly unaffected by maternal diet.
TABLE 4.
Maternal hepatic uracil content in genomic DNA1
| Maternal genotype | Liver uracil | |
| pg uracil/μg DNA | ||
| AIN93G | Shmt1+/+ | 0.69 ± 0.23 |
| Shmt1−/− | 0.66 ± 0.25 | |
| AIN93G minus choline | Shmt1+/+ | 0.92 ± 0.28 |
| Shmt1−/− | 0.93 ± 0.25 | |
| AIN93G minus folate | Shmt1+/+ | 0.65 ± 0.25 |
| Shmt1−/− | 0.84 ± 0.25 | |
| P value | ||
| Diet effect | NS | |
| Genotype effect | NS | |
| Diet × genotype effect | NS | |
All values are means ± SEMs. n = 6 per group. Differences between Shmt1 genotypes, diets, and the interaction of genotype and diet were analyzed by 2-factor ANOVA by using Tukey's honestly significant difference post hoc analysis. AIN93G diet (Dyets).
DISCUSSION
We have recently reported that embryonic SHMT1 deficiency causes NTDs in response to combined maternal dietary folate and choline deficiency (13). Here we show that maternal folate deficiency alone was sufficient to induce NTDs in response to embryonic Shmt1 disruption and that dietary choline deficiency did not contribute to NTD risk in this mouse model. In this study, we observed a frequency of NTDs (33%) in Shmt1−/− embryos derived from dams maintained on a diet deficient only in folate similar to what we observed previously when the same mice were fed a diet deficient in both folate and choline (22%) (13). In contrast, NTDs were not observed in Shmt1−/− embryos when pregnant dams were maintained on a diet deficient only in choline. These data indicate that in the original study of the SHMT1-deficient mouse model, choline deficiency did not exacerbate the effect of folate deficiency on NTD pathogenesis (13). Indeed, dietary choline deficiency did not affect plasma folate status in this study, and conversely, Shmt1 disruption and folate deficiency did not interact to alter markers of choline metabolism. Choline deficiency similarly did not affect embryonic growth in litters from Shmt1−/− mice, as was observed under folate-deficient conditions. However, it is important to note that the gut microflora remained intact in these studies and likely contributed to both folate and choline nurtriture in mice and therefore prevented severe deficiency (19). Consistent with a previous report (13), no NTDs were observed in Shmt1+/+ embryos, regardless of maternal dietary manipulation. Although embryonic genotype was confounded by maternal genotype in this study, we have previously shown that maternal genotype does not significantly influence NTD risk (13). Together, these data confirm that NTDs in the SHMT1-deficient mouse model result from maternal dietary folate, but not choline, deficiency.
Folate and choline metabolism intersect at the level of homocysteine remethylation and the consequent availability of methyl groups for cellular methylation reactions (Figure 1); choline status may influence NTD risk by affecting homocysteine concentrations and cellular methylation or by mechanisms unrelated to homocysteine and/or folate metabolism, including membrane biosynthesis (10). The SHMT1-deficient mouse represents the first folate-responsive NTD mouse model with a genetic disruption in the folate metabolism pathway (13). This gene-diet interaction closely resembles the interactions found in human studies of NTD pathogenesis (3, 4) and indicates a disruption in thymidylate biosynthesis (13). Thus, the absence of NTDs in the SHMT1-deficient mouse in response to dietary choline deficiency suggests that the increased risk of NTDs associated with dietary choline deficiency in humans may be unrelated to a disruption of folate metabolism. Further investigation of the mechanisms by which choline deficiency increases risk of NTDs in humans is warranted.
If elevated homocysteine concentrations, disrupted cellular methylation, or both were a cause of NTDs in the SHMT1-deficient mouse model, it would be expected that dietary choline deficiency would exacerbate or increase NTD incidence. Reduced concentrations of betaine and dimethylglycine indicate that the CD diet curtailed the use of choline as a methyl donor, a finding consistent with previous reports (20). The lack of an effect of dietary choline deficiency on NTD frequency and severity in SHMT1-deficient embryos in the present study provides another line of evidence that homocysteine remethylation does not play a causal role in the pathogenesis of folate-responsive NTDs. Although pharmacologic disruption of choline metabolism has been shown to impair neurulation in cultured embryos (11), to our knowledge there has been no report of dietary choline deficiency resulting in NTDs in vivo. Similarly, choline deficiency does not cause NTDs in mice deficient for MTHFR (methylenetetrahydrofolate reductase), a folate-dependent enzyme required for homocysteine remethylation, despite highly elevated homocysteine concentrations (21). Together, these data provide additional support for the hypothesis that disrupted homocysteine remethylation is not an underlying cause of pathogenesis in folate-responsive NTDs.
SHMT1 preferentially shuttles one-carbon units toward thymidylate synthesis (Figure 1). We have recently reported that DNA isolated from livers of male Shmt1+/−, but not Shmt1−/−, mice exhibits increased uracil content compared with Shmt1+/+ mice (14, 22). In the present study, we did not observe any effect of diet or genotype on liver uracil content between Shmt1+/+ and Shmt1−/− pregnant dams. Previously, we showed that both thymidylate synthase and the salvage pathway for thymidine nucleotides are upregulated and reduce genomic uracil misincorporation in Shmt1−/− mice, but not in Shmt1+/− mice (22). In contrast, the embryo relies primarily on de novo nucleotide biosynthesis with limited capacity for salvage pathway synthesis (23). The findings from this study therefore suggest that maternal folate deficiency may affect NTD outcomes indirectly by exacerbating impairments in thymidylate biosynthesis occurring at the level of the embryo in Shmt1−/− mice.
Acknowledgments
We thank Martha Field for thoughtful insight and discussion and Francoise Vermeylen for assistance with statistical analysis.
The authors’ responsibilities were as follows—AEB and EVA: study conception and design, data collection and analysis, and manuscript preparation, editing, and revision; CAP and OM: data collection; MC: study design and manuscript preparation and editing; and PJS: study conception and design and manuscript editing and revision. There were no conflicts of interest for any of the authors.
Footnotes
Abbreviations used: AdoMet, S-adenosylmethionine; CD, AIN93G diet deficient in choline; CR, crown-rump; E, embryonic day; FCD, AIN93G diet lacking both choline and folate; FD, AIN93G diet deficient in folate; NTD, neural tube defect; SHMT1, serine hydroxymethyltransferase 1.
REFERENCES
- 1.DeSesso JM, Scialli AR, Holson JF. Apparent lability of neural tube closure in laboratory animals and humans. Am J Med Genet 1999;87:143–62 [DOI] [PubMed] [Google Scholar]
- 2.Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 1993;86:703–8 [PubMed] [Google Scholar]
- 3.Relton CL, Wilding CS, Laffling AJ, Jonas PA, Burgess T, Binks K, Tawn EJ, Burn J. Low erythrocyte folate status and polymorphic variation in folate-related genes are associated with risk of neural tube defect pregnancy. Mol Genet Metab 2004;81:273–81 [DOI] [PubMed] [Google Scholar]
- 4.Christensen B, Arbour L, Tran P, Leclerc D, Sabbaghian N, Platt R, Gilfix BM, Rosenblatt DS, Gravel RA, Forbes P, et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet 1999;84:151–7 [DOI] [PubMed] [Google Scholar]
- 5.Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm 2008;79:1–44 [DOI] [PubMed] [Google Scholar]
- 6.Lipton SA, Kim WK, Choi YB, Kumar S, D'Emilia DM, Rayadu PV, Arnell DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA 1997;94:5923–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol 2004;160:102–9 [DOI] [PubMed] [Google Scholar]
- 8.Shaw GM, Finnell RH, Blom HJ, Carmichael SL, Vollset SE, Yang W, Ueland PM. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology 2009;20:714–9 [DOI] [PubMed] [Google Scholar]
- 9.Zeisel SH. Dietary choline: biochemistry, physiology, and pharmacology. Annu Rev Nutr 1981;1:95–121 [DOI] [PubMed] [Google Scholar]
- 10.Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr 2009;89:673S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology 2001;64:114–22 [DOI] [PubMed] [Google Scholar]
- 12.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J 2002;16:619–21 [DOI] [PubMed] [Google Scholar]
- 13.Beaudin AE, Abarinov EV, Noden DM, Perry CY, Chu S, Stabler SP, Allen RH, Stover PJ. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am J Clin Nutr 2011;93:789–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacFarlane AJ, Liu X, Perry CA, Flodby P, Allen RH, Stabler SP, Stover PJ. Cytoplasmic serine hydroxymethyltransferase regulates the metabolic partitioning of methylenetetrahydrofolate but is not essential in mice. J Biol Chem 2008;283:25846–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClive PJ, Sinclair AH. Rapid DNA extraction and PCR-sexing of mouse embryos. Mol Reprod Dev 2001;60:225–6 [DOI] [PubMed] [Google Scholar]
- 16.Suh JR, Oppenheim EW, Girgis S, Stover PJ. Purification and properties of a folate-catabolizing enzyme. J Biol Chem 2000;275:35646–55 [DOI] [PubMed] [Google Scholar]
- 17.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 2003;49:286–94 [DOI] [PubMed] [Google Scholar]
- 18.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 2002;74:4734–40 [DOI] [PubMed] [Google Scholar]
- 19.Walzem RL, Clifford AJ. Folate deficiency in rats fed diets containing free amino acids or intact proteins. J Nutr 1988;118:1089–96 [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Agellon LB, Vance DE. Phosphatidylcholine homeostasis and liver failure. J Biol Chem 2005;280:37798–802 [DOI] [PubMed] [Google Scholar]
- 21.Chan J, Deng L, Mikael LG, Yan J, Pickell L, Wu Q, Caudill MA, Rozen R. Low dietary choline and low dietary riboflavin during pregnancy influence reproductive outcomes and heart development in mice. Am J Clin Nutr 2010;91:1035–43 [DOI] [PubMed] [Google Scholar]
- 22.MacFarlane AJ, Perry CA, McEntee MF, Lin DM, Stover PJ. Shmt1 heterozygosity impairs folate-dependent thymidylate synthesis capacity and modifies risk of Apc(min)-mediated intestinal cancer risk. Cancer Res 2011;71:2098–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe PB, McEwen SE. De novo purine synthesis in cultured rat embryos undergoing organogenesis. Proc Natl Acad Sci USA 1983;80:7333–6 [DOI] [PMC free article] [PubMed] [Google Scholar]