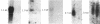Abstract
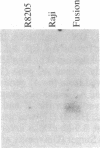
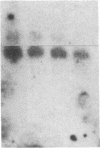
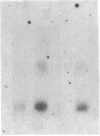
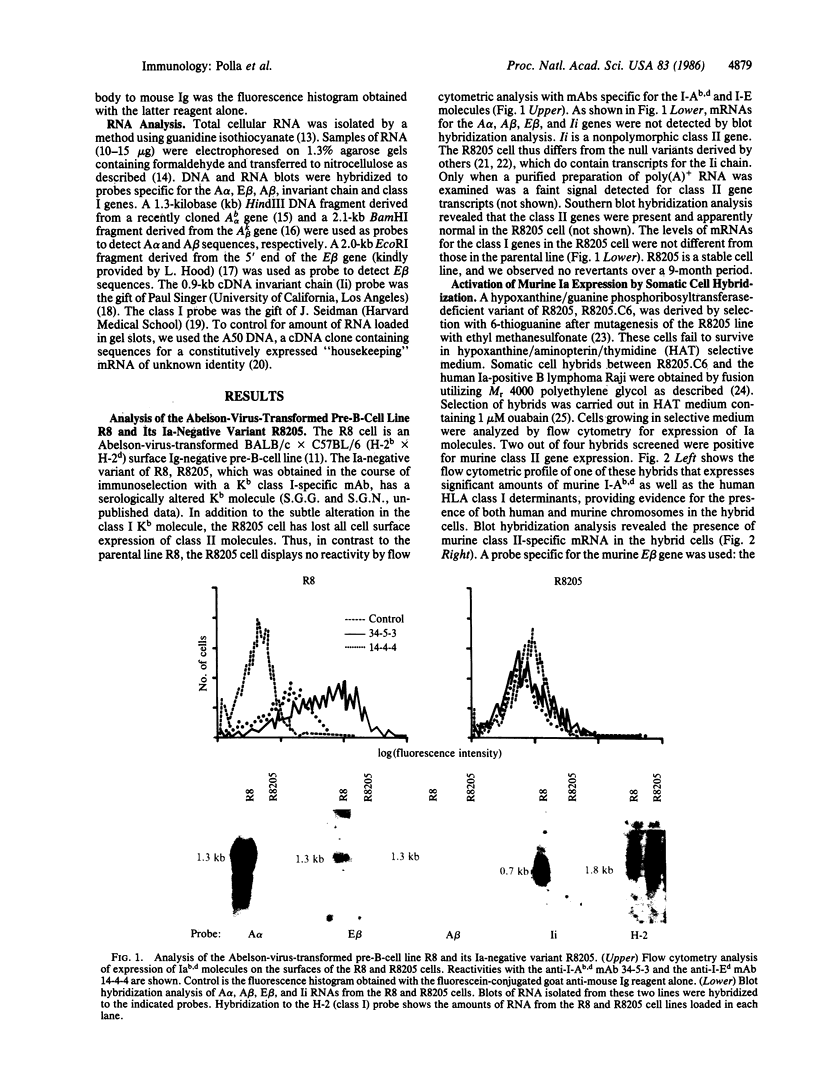
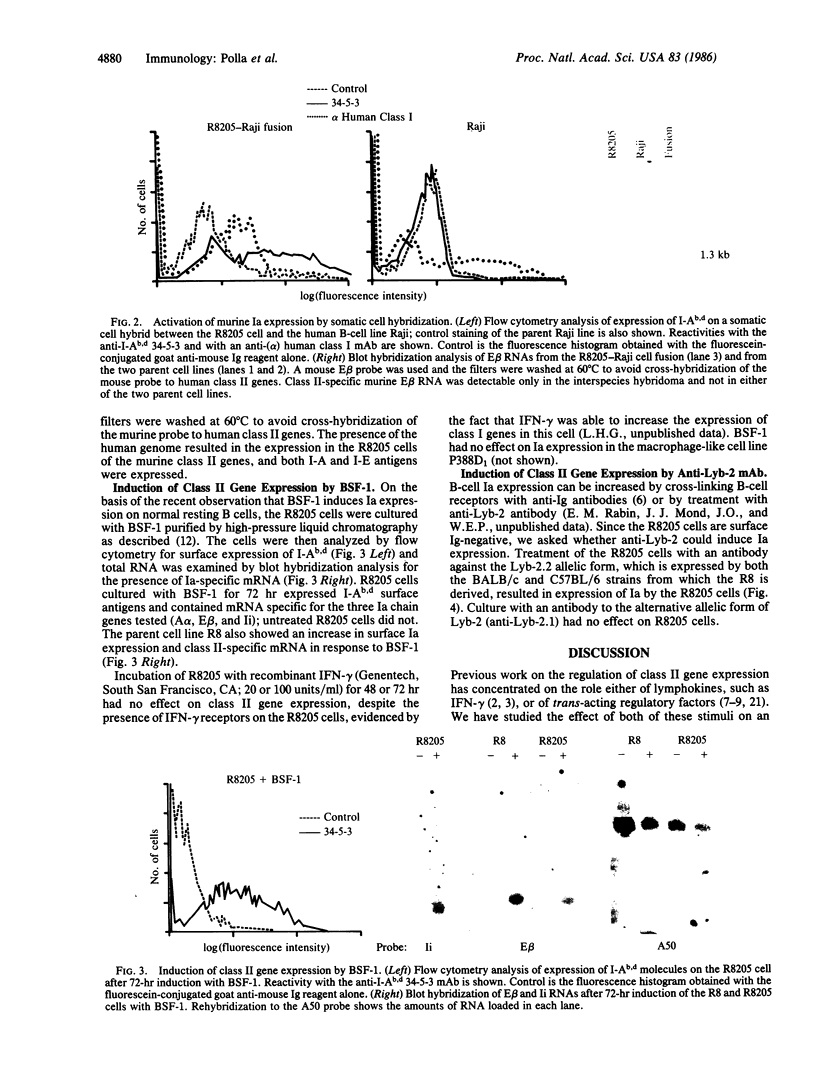
Expression of class II genes of the major histocompatibility complex (MHC) has been studied in an Abelson-murine-leukemia-virus-transformed pre-B-cell line, R8, and its class II molecule (Ia)-negative variant, R8205. These variant cells contained barely detectable levels of RNA specific for all class II genes, including the nonpolymorphic invariant chain gene (Ii), and did not express cell surface Ia. Fusion of this murine Ia-negative cell line to the human Ia-positive Raji cell produced an interspecies hybridoma that expressed the murine Ia. These data are further evidence for the existence of a trans-acting factor(s) that can regulate class II gene expression. Furthermore, the T-cell-derived lymphokine B-cell-stimulatory factor 1 (BSF-1) induced expression of class II genes in the R8205 cells. Exposure of R8205 cells to an antibody that has been shown to mimic BSF-1 activity on normal B cells also resulted in expression of class II genes. These data demonstrate that three distinct signals--a lymphokine, an alloantibody binding to membrane structures, and an interspecies trans-acting factor--can induce expression of class II genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Carra G., Buchegger F., Carrel S., Mach J. P. The human Ia-associated invariant chain is synthesized in Ia-negative B cell variants and is not expressed on the cell surface of both Ia-negative and Ia-positive parental cells. J Immunol. 1985 May;134(5):3265–3271. [PubMed] [Google Scholar]
- Accolla R. S., Carra G., Guardiola J. Reactivation by a trans-acting factor of human major histocompatibility complex Ia gene expression in interspecies hybrids between an Ia-negative human B-cell variant and an Ia-positive mouse B-cell lymphoma. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5145–5149. doi: 10.1073/pnas.82.15.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Ben-Nun A., Choi E., McIntyre K. R., Leeman S. A., McKean D. J., Seidman J. G., Glimcher L. H. DNA-mediated transfer of major histocompatibility class II I-Ab and I-Abm12 genes into B lymphoma cells: molecular and functional analysis of introduced antigens. J Immunol. 1985 Aug;135(2):1456–1464. [PubMed] [Google Scholar]
- Ben-Nun A., Glimcher L. H., Weis J., Seidman J. G. Functional expression of a cloned I-A beta k gene in B-lymphoma cells. Science. 1984 Feb 24;223(4638):825–828. doi: 10.1126/science.6420890. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Gladstone P., Pious D. Identification of a trans-acting function regulation HLA-DR expression in a DR-negative B cell variant. Somatic Cell Genet. 1980 Mar;6(2):285–298. doi: 10.1007/BF01538802. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., Hamano T., Asofsky R., Sachs D. H., Pierres M., Samelson L. E., Sharrow S. O., Paul W. E. IA mutant functional antigen-presenting cell lines. J Immunol. 1983 May;130(5):2287–2294. [PubMed] [Google Scholar]
- Glimcher L. H., Shevach E. M. Production of autoreactive I region-restricted T cell hybridomas. J Exp Med. 1982 Aug 1;156(2):640–645. doi: 10.1084/jem.156.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiya M., Davis D. D., Takahashi T., Okuda K., Raschke W. C., Sakano H. Two types of immunoglobulin-negative Abelson murine leukemia virus-transformed cells: implications for B-lymphocyte differentiation. Proc Natl Acad Sci U S A. 1986 Jan;83(1):145–149. doi: 10.1073/pnas.83.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine F., Erlich H. A., Mach B., Pious D. Transcriptional regulation of HLA class II and invariant chain genes. J Immunol. 1985 Jan;134(1):637–640. [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Seghal E., Kung J., Finkelman F. D. Increased expression of I-region-associated antigen (Ia) on B cells after cross-linking of surface immunoglobulin. J Immunol. 1981 Sep;127(3):881–888. [PubMed] [Google Scholar]
- Nguyen H. T., Medford R. M., Nadal-Ginard B. Reversibility of muscle differentiation in the absence of commitment: analysis of a myogenic cell line temperature-sensitive for commitment. Cell. 1983 Aug;34(1):281–293. doi: 10.1016/0092-8674(83)90159-9. [DOI] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Oliver K., Noelle R. J., Uhr J. W., Krammer P. H., Vitetta E. S. B-cell growth factor (B-cell growth factor I or B-cell-stimulating factor, provisional 1) is a differentiation factor for resting B cells and may not induce cell growth. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2465–2467. doi: 10.1073/pnas.82.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. M., Ohara J., Paul W. E. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter R. D., Alexander J., Levine F., Pious D., Cresswell P. Evidence for two trans-acting genes regulating HLA class II antigen expression. J Immunol. 1985 Dec;135(6):4235–4238. [PubMed] [Google Scholar]
- Singer P. A., Lauer W., Dembić Z., Mayer W. E., Lipp J., Koch N., Hämmerling G., Klein J., Dobberstein B. Structure of the murine Ia-associated invariant (Ii) chain as deduced from a cDNA clone. EMBO J. 1984 Apr;3(4):873–877. doi: 10.1002/j.1460-2075.1984.tb01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Schaefer M. E., Finkelman F. D., Scher I., Farrar J., Mond J. J. T cell-derived B cell growth factor(s) can induce stimulation of both resting and activated B cells. J Immunol. 1985 Jan;134(1):369–374. [PubMed] [Google Scholar]
- Weis J. H., Seidman J. G. The expression of major histocompatibility antigens under metallothionein gene promoter control. J Immunol. 1985 Mar;134(3):1999–2003. [PubMed] [Google Scholar]
- Zlotnik A., Shimonkevitz R. P., Gefter M. L., Kappler J., Marrack P. Characterization of the gamma-interferon-mediated induction of antigen-presenting ability in P388D1 cells. J Immunol. 1983 Dec;131(6):2814–2820. [PubMed] [Google Scholar]
- de Préval C., Lisowska-Grospierre B., Loche M., Griscelli C., Mach B. A trans-acting class II regulatory gene unlinked to the MHC controls expression of HLA class II genes. Nature. 1985 Nov 21;318(6043):291–293. doi: 10.1038/318291a0. [DOI] [PubMed] [Google Scholar]