Abstract
Background: Children with edematous severe acute malnutrition (SAM) produce less cysteine than do their nonedematous counterparts. They also have marked glutathione (GSH) depletion, hair loss, skin erosion, gut mucosal atrophy, and depletion of mucins. Because GSH, skin, hair, mucosal, and mucin proteins are rich in cysteine, we hypothesized that splanchnic extraction and the efficiency of cysteine utilization would be greater in edematous than in nonedematous SAM.
Objective: We aimed to measure cysteine kinetics in childhood edematous and nonedematous SAM.
Design: Cysteine flux, oxidation, balance, and splanchnic uptake (SPU) were measured in 2 groups of children with edematous (n = 9) and nonedematous (n = 10) SAM at 4.4 ± 1.1 d after admission (stage 1) and at 20.5 ± 1.6 d after admission (stage 2) when they had replenished 50% of their weight deficit.
Results: In comparison with the nonedematous group, the edematous group had slower cysteine flux at stage 1 but not at stage 2; furthermore, they oxidized less cysteine at both stages, resulting in better cysteine balance and therefore better efficiency of utilization of dietary cysteine. Cysteine SPU was not different between groups but was ∼45% in both groups at the 2 stages.
Conclusion: These findings suggest that children with edematous SAM may have a greater requirement for cysteine during early and mid–nutritional rehabilitation because they used dietary cysteine more efficiently than did their nonedematous counterparts and because the splanchnic tissues of all children with SAM have a relatively high requirement for cysteine. This trial was registered at clinicaltrials.gov as NCT00069134.
INTRODUCTION
In earlier studies of GSH4 metabolism in children with severe childhood undernutrition (SAM), we found that slower erythrocyte GSH synthesis in those with edema was associated with lower concentrations of cysteine, the rate-limiting precursor of GSH synthesis (1). The increase in GSH synthesis brought about by cysteine supplementation during the early phase of nutritional rehabilitation suggested a shortage in cysteine availability in children with edematous SAM (2). In more recent studies we reported that the fluxes of cysteine and its precursor methionine were slower in edematous SAM than in nonedematous SAM because of decreased release from a slower whole-body protein breakdown rate (3, 4). Because both amino acids are needed for protein synthesis, cysteine is the rate-limiting amino acid for GSH synthesis, methionine is required for initiation of protein synthesis and is a precursor for the synthesis of polyamines and S-adenosylmethionine, and both amino acids are required in relatively larger proportions for synthesis of collagen and the keratin of skin and hair. Roediger (5) proposed that a profound shortage in the availability of sulfur amino acids during a time of greatest requirement (ie, early childhood) may be responsible for some of the signs and symptoms of edematous SAM.
In addition, because gut mucosal proteins and mucins are rich in cysteine (6), and they turnover at extremely fast rates, ∼100–140% per day (7, 8), cysteine deficiency may be contributing significantly to the intestinal mucosal atrophy and reduced secretory mucins, and therefore to the impaired gut function and leakiness, observed in children with SAM (9). These structural and functional abnormalities will be more pronounced in those with edematous SAM on the basis of the evidence of more severe gut mucosal atrophy observed in this group than in children with nonedematous SAM (10). There is strong evidence from animal studies that cysteine and GSH play key roles in maintaining intestinal structure and function (11–13). Therefore, during early nutritional rehabilitation the requirement for cysteine to repair the gut will be greater in children with edematous SAM than in those with nonedematous SAM. Furthermore, children with edematous SAM should use dietary cysteine more efficiently to meet overall needs because of decreased availability from body protein breakdown.
We tested 2 hypotheses. First, the efficiency of utilization of cysteine will be much greater in children with edematous SAM than in nonedematous SAM during the early (stage 1) and mid-CUG (stage 2) stages of nutritional rehabilitation. Second, because of the need to repair and replenish gut tissues and secretory mucins early, the splanchnic extraction of cysteine will be greater in edematous SAM at stage 1 but not at stage 2. To test these hypotheses we used a dual stable-isotope-tracer method to measure cysteine flux and its oxidation and splanchnic extraction in the fed state at stages 1 and 2 of rehabilitation.
SUBJECTS AND METHODS
Subjects
Nineteen children, aged 4–24 mo, who were admitted to the Tropical Metabolism Research Unit of the University of the West Indies, Jamaica, for treatment of primary SAM were recruited. On the basis of the Wellcome classification (14), they were assigned to either an edematous group (kwashiorkor and marasmic-kwashiorkor) or to a nonedematous group (marasmus and undernutrition). Nine children had edematous SAM (4 boys, 5 girls), 3 with kwashiorkor and 6 with marasmic kwashiorkor; 10 children had nonedematous SAM (6 boys, 4 girls), 3 with marasmus and 7 with undernutrition. Treatment followed a standard protocol as described previously (15). This study was conducted according to the guidelines laid down by the Declaration of Helsinki. The University Hospital/University of the West Indies Faculty of Medical Sciences Ethics Committee and the Baylor Affiliates Review Board for Human Subject Research, Baylor College of Medicine, approved the study. Written informed consent was obtained from a parent or guardian of each child enrolled.
Study design
The study consisted of a group of 9 children with edematous SAM and a group of 10 children with nonedematous SAM. By using stable-isotope-tracer methodology, cysteine kinetics were measured at 4.4 ± 1.1 d after admission in the fed state when the subjects were severely malnourished but clinically stable as indicated by blood pressure, pulse, and respiration rates (stage 1 experiment) and at 20.5 ± 1.6 d after admission (stage 2 experiment) during the mid-CUG phase when they had replenished 50% of their weight deficit. During the stage 1 measurement, the children were fed a weight-maintenance diet intragastrically, and a high-energy diet was fed during the stage 2 measurement. These diets are based on a standard dietary treatment protocol that takes into consideration the metabolic capacity at different stages of rehabilitation and was previously described by us (1, 2, 16). During the stage 1 experiment, all of the children received the same amount of maintenance diet to provide 417 kJ · kg−1 · d−1 and 1.2 g protein · kg−1 · d−1, but during the stage 2 experiment they were fed on the basis of their individual intake at that time. At this time, the children were consuming ∼655 kJ · kg−1 · d−1 and ∼3.4 g protein · kg−1 · d−1. On the experiment day, intragastric feeding started 2 h before the start of an 8-h isotope infusion. Therefore, 42% of the subject's daily food intake was provided by constant intragastric infusion over a 10-h period. Intravenous catheters were placed in each arm, one for infusion of isotopes and one for withdrawal of blood samples. First, isotopically labeled sodium bicarbonate was infused intravenously and breath samples collected for measuring carbon dioxide production, followed by simultaneous intravenous and intragastric infusions of 2 different isotopes of cysteine to measure cysteine kinetics.
Weight and length were measured daily with an electronic balance (model F150S; Sartorius) and with a horizontal mounted stadiometer (Holtain Ltd), respectively. The mean rate of weight gain over 4 consecutive days during the mid-CUG phase was calculated starting 2 d before the isotope study.
Isotopic tracer infusion protocol
After 2 h of intragastric feeding, breath and blood (0.5 mL) samples were collected followed by a primed (P) continuous infusion (I) of NaH13CO3 (P = 4.5 μmol/kg at stage 1 and 6 μmol/kg at stage 2, I = 6 μmol · kg−1 · h−1) for 2 h. More breath samples were collected every 15 min in the second hour. After 2 h, the NaH13CO3 was stopped and an intragastric infusion of 2H2-cysteine (P = 4 μmol/kg, I = 3 μmol · kg−1 · h−1) and an intravenous infusion of U-13C3-cysteine (P = 3.75 μmol/kg, I = 3 μmol· kg−1 · h−1) were started simultaneously and maintained for 6 h. During the last 2 h of infusion, 4 more blood and breath samples were obtained: the first 2 were collected hourly and the last 2 at 0.5-h intervals.
Sample analyses
Blood was collected in heparinized tubes and centrifuged immediately at 1000 g for 15 min at 4°C; the plasma removed and stored immediately at −70°C for later analyses. The plasma cysteine tracer:tracee ratios were measured by using a triple quadrupole mass spectrometer (TSQ Vantage; Thermo Scientific), equipped with an HESI (heated-electrospray ionization) source, an Accela pump (Thermo Scientific), and a Thermal PAL autosampler (Thermo Scientific). Dithiothreitol (60 mmol/L in 0.1 mol sodium tetraborate/L) was added to the plasma sample to convert cystine to cysteine. Cysteine was then alkylated by adding iodoacetamide (0.5 mol/L) in 0.1 mol ammonium bicarbonate/L. Alkylated cysteine was converted into its DANS [5-(dimethylamino)-1-napthalene sulfonamide] derivative and analyzed by liquid chromatography–mass spectrometry on a Phenomenex Synergi MAX-RP 4 μm 150 × 2.0 mm column. The ions were then analyzed by selected reaction monitoring mode. The transitions observed were precursor ions m/z 412, 414, and 415 to product ion m/z 170 at 27 electron volts. Instrumental control and data acquisition and analysis were performed by the XCalibur (version 2.1) software package (Thermo Scientific).
Total plasma cysteine (cystine plus cysteine) concentration was measured by in vitro isotope dilution. A known quantity of U-13C3-cysteine (Cambridge Isotope Laboratories) was added as an internal standard to the baseline plasma samples; dithiothreitol (60 mmol/L in 0.1 mol sodium tetraborate/L) was added to convert cystine to cysteine, and the sample was processed as described above.
The breath samples were analyzed for 13C abundance in carbon dioxide by gas isotope ratio–mass spectrometry (ThermoQuest Finnigan Deltaplus XL Isotope Ratio Mass Spectrometer coupled with Gasbench-II; Thermo Scientific) monitoring of ions at m/z 44 and 45.
Calculations
The following standard steady state equation was used to calculate total flux (Q) of cysteine and carbon dioxide:
where Tr/trinf is the tracer:tracee ratio of the U-13C-cysteine (or of the NaH13CO3) infusate and Tr/trplat is the tracer:tracee ratio of cysteine (M+3 isotopomer) in plasma (or 13CO2 in expired air) at plateau and iIV is the intravenous infusion rate of the respective tracer in μmol · kg−1 · h−1.
Endogenous flux was calculated as the difference between total flux and the intravenous tracer in the case of carbon dioxide and the tracer infusions plus dietary intake in the case of cysteine.
Cysteine oxidation rate was calculated by using the following equation:
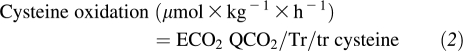 |
where ECO2 and QCO2 are the isotopic enrichment and flux of carbon dioxide and Tr/tr cysteine is the steady state tracer:tracee ratio of the m+3 isotopomer of cysteine.
Cysteine balance (μmol · kg−1 · h−1) was calculated as total intake minus oxidation. Efficiency of utilization of exogenous cysteine was calculated as cysteine balance expressed as percentage of total intake.
The percentage of dietary cysteine extracted by the splanchnic tissues (%CYSsplan) was obtained from the following equation:
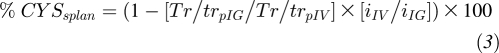 |
where Tr/trpIG and Tr/trpIV are the steady state tracer-to-tracee ratios of the intragastric (IG) and intravenous (IV) tracers and iIG and iIV are the rates of infusion of the IG and IV tracers.
SPU was calculated as the product of %CYSsplan and enteral cysteine intake, ie, dietary cysteine plus the IG-infused cysteine tracer.
The kinetic and anthropometric data in the stage 1 experiments were expressed per kilogram of edema-free body weight. Edema weight was estimated as the difference between body weight on the day of the stage 1 experiment and the lowest postexperiment weight observed. During this period, all patients were fed the maintenance diet, which does not promote weight gain. The edematous children lost their edema before the stage 2 experiment.
Statistical analyses
Differences in the physical and clinical characteristics of the 2 groups at each stage were determined by nonpaired 2-tailed t test. The kinetic data were analyzed by using 2-factor repeated-measures ANOVA, with the between-group factor being the diagnosis and the repeated factor being the stage measurement over time [stage 1 (acutely malnourished) to stage 2 (mid-CUG)]. Stata statistical software version 8 for Windows (Stata Corporation) was used for the analysis. Results were considered to be significant if P < 0.05.
RESULTS
Physical characteristics of the subjects are shown in Table 1. Age was not significantly different between the 2 groups. At stage 1, although the children in nonedematous group were significantly longer (P = 0.005) and heavier (P = 0.03) than in the edematous group, they were more wasted as indicated by lower weight for length (P = 0.015). At stage 1, both nonedematous and edematous children were hypoalbuminemic, but albumin concentrations were significantly lower in the edematous compared with the nonedematous children (P < 0.001). At stage 2, except for 3 children with edematous SAM, albumin concentrations of the children in both groups were within the normal range. At both stages 1 and 2, hemoglobin concentrations in the edematous group were lower than in the nonedematous group (P = 0.004 and 0.04, respectively). All except 2 of the nonedematous children had 1–4 types of infection (Table 2). All of the infections resolved with broad-spectrum antibiotic treatment within the first 10 d of treatment. Therefore, all children had been free of infections for ∼10 d when the stage 2 experiment was performed.
TABLE 1.
Physical and clinical characteristics of the subjects1
| Stage 1 |
Stage 2 |
|||
| Characteristic | Nonedematous | Edematous | Nonedematous | Edematous |
| n | 10 | 9 | 10 | 9 |
| Age (mo) | 13.0 ± 2.1 | 8.7 ± 1.3 | 13.5 ± 2.12 | 9.4 ± 1.32 |
| Weight (kg)3 | 6.0 ± 0.4 | 4.7 ± 0.44 | 6.7 ± 0.52 | 5.2 ± 0.324 |
| Length (cm) | 67.7 ± 2.2 | 59.2 ± 1.44 | 68.4 ± 2.22 | 59.4 ± 1.34 |
| Weight for age3 (% of expected) | 61.7 ± 3.4 | 55.9 ± 4.3 | 67.7 ± 3.72 | 59.4 ± 3.624 |
| Weight for length3 (% of expected) | 77.6 ± 1.7 | 86.3 ± 2.84 | 84.8 ± 1.92 | 93.5 ± 1.62 |
| Albumin (g/L) | 37.4 ± 2.0 | 20.1 ± 2.44 | 42.9 ± 1.42 | 33.0 ± 3.24 |
| Hemoglobin (g/dL) | 9.5 ± 0.4 | 7.7 ± 0.44 | 9.5 ± 0.3 | 8.6 ± 0.324 |
All values are means ± SEMs. Stage 1: ∼4 d after admission; stage 2: ∼20 d after admission when the children had replaced 50% of their weight deficit.
Significantly different from the corresponding clinical phase, P < 0.05 (paired t test).
Weight was adjusted for edema which was estimated as the difference between body weight on the day of the stage 1 experiment and the lowest post experiment weight measured. All children were edema free at stage 2.
Significantly different from nonedematous patients in the same clinical phase, P < 0.05 (unpaired t test).
TABLE 2.
Type of infection, temperature, and WBC count of subjects at admission1
| Variable | Edematous | Nonedematous |
| Sex (M/F) | 4:5 | 6:4 |
| Type of infection | 1 B, 5 C, 1 D, 2 LRTI, 3 OM, 2 URTI, 2 UTI | 2 B, 1 C, 2 D, 2 LRTI, 3 OM, 5 URTI, 1 UTI |
| WBC count (1 × 109/L)2 | 11.8 ± 1.3 | 15.5 ± 3.1 |
| Temperature (°C)2 | 37 ± 0 | 37 ± 0 |
B, bacteremia; C, candidiasis; D, infective diarrhea; LRTI, lower respiratory tract infection; OM, otitis media; URTI, upper respiratory tract infection; UTI, urinary tract infection; WBC, white blood cell.
Values are means ± SEMs. There were no differences between the edematous and nonedematous groups of children (nonpaired, 2-tailed t test).
Cysteine intake and SPU are shown in Table 3. There was no difference in dietary or total exogenous cysteine intake between the groups at stage 1 or at stage 2. Dietary intake was greater at stage 2 than at stage 1 in both groups (P < 0.01). Similarly, there was a significant effect of clinical stage because the amount of cysteine extracted by the splanchnic bed was greater at stage 2 (P < 0.01) in both groups. There was no significant clinical stage by diagnosis group interaction in SPU because the fraction of cysteine extracted by the splanchnic bed was not different between the groups or between stages.
TABLE 3.
Cysteine intake and splanchnic uptake in children diagnosed with nonedematous and edematous severe acute malnutrition during rehabilitation1
| Stage 1 |
Stage 2 |
|||
| Nonedematous (n = 10) | Edematous (n = 9) | Nonedematous (n = 10) | Edematous (n = 9) | |
| Dietary cysteine2 (μmol · kg−1 · h−1) | 7.8 ± 0.3 | 8.1 ± 0.2 | 19.8 ± 0.5 | 20.5 ± 0.4 |
| Total intragastric cysteine 23 (μmol · kg−1 · h−1) | 10.9 ± 0.3 | 11.3 ± 0.2 | 22.8 ± 0.5 | 23.5 ± 0.4 |
| Total exogenous cysteine 24 (μmol · kg−1 · h−1) | 13.9 ± 0.3 | 14.4 ± 0.3 | 25.8 ± 0.5 | 26.5 ± 0.4 |
| Fraction of splanchnic uptake | 0.47 ± 0.04 | 0.51 ± 0.05 | 0.44 ± 0.03 | 0.43 ± 0.05 |
| Splanchnic uptake 2 (μmol · kg−1 · h−1) | 5.10 ± 0.43 | 5.46 ± 0.75 | 10.02 ± 0.71 | 10.10 ± 1.22 |
All values are means ± SEMs. Stage 1: ∼4 d after admission; stage 2: ∼20 d after admission when the children were growing rapidly and had replaced 50% of their weight deficit. Values were compared by repeated-measures 2-factor ANOVA.
Main effect of stage, P < 0.01.
Sum of dietary cysteine and intragastric tracer cysteine.
Sum of dietary cysteine and intragastric and intravenous tracer cysteine.
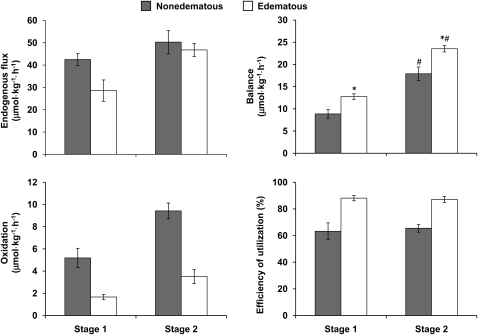
Endogenous cysteine flux, oxidation, balance, and efficiency of utilization are shown in Figure 1. At stage 1 there was a significant effect of diagnosis because endogenous cysteine flux was slower in the edematous children with SAM than in the nonedematous children (P < 0.02). Flux increased significantly from stages 1 to 2 in the edematous group (main effect of clinical stage, P < 0.01) but not in the nonedematous group. At stage 2, there was no difference between the groups.
FIGURE 1.
Mean (±SEM) cysteine endogenous flux, balance, oxidation, and efficiency of utilization in children diagnosed with nonedematous (shaded bars; n = 10) and edematous (open bars; n = 9) severe childhood malnutrition at stage 1, ∼4 d after admission, when severely malnourished, and at stage 2 ∼20 d after admission when the children had replaced 50% of their weight deficit. Analyses were performed by using repeated-measures ANOVA. Flux: main effect of diagnosis and stage terms, P < 0.02; stage-by-diagnosis interaction, P = 0.4. Balance: *Significantly different from nonedematous patients in the same clinical phase, P < 0.05. #Significantly different from corresponding clinical phase, P < 0.05; stage-by-diagnosis interaction, P < 0.02. Oxidation: main effect of diagnosis and stage terms, P < 0.001; stage-by-diagnosis interaction, P = 0.05. Efficiency of cysteine utilization: main effect of diagnosis term, P < 0.03; stage-by-diagnosis interaction, P = 0.42.
With respect to cysteine oxidation, there was a trend toward a significant stage by diagnosis group interaction (P = 0.05) because oxidation increased by a much greater magnitude in the nonedematous group than in the edematous group. Both diagnosis and clinical stage had significant main effects. Cysteine oxidation rates increased significantly from stage 1 to stage 2 in both groups (P < 0.001) and were significantly slower (P < 0.001) in the children with edematous SAM compared with the values of those with nonedematous SAM at both clinical stages. As a consequence, cysteine balance was significantly greater (P < 0.05) in the children with edematous SAM at both stages. Similarly, the efficiency of utilization of dietary cysteine was also significantly greater (P < 0.001) in the children with edematous SAM at both stages.
Despite the better cysteine balance at both stages, total plasma cysteine concentration (cystine plus cysteine) was significantly lower (P < 0.05) in the edematous children than in the nonedematous children (stage 1: 72.5 ± 15.0 compared with 189.0 ± 14.5 μmol/L, respectively; stage 2: 168.3 ± 14.1 compared with 207.6 ± 10.1 μmol/L, respectively). The edematous children had significantly higher total plasma cysteine concentration at stage 2 than at stage 1 (P < 0.05).
DISCUSSION
In this study we tested the hypotheses that the efficiency of utilization of cysteine will be much greater in children diagnosed with edematous SAM than in those with nonedematous SAM during the acutely malnourished and mid-CUG stages of nutritional rehabilitation and that cysteine SPU will be much greater in children with edematous SAM but not at mid-CUG. In comparison with the children with nonedematous SAM, the children with edematous SAM had slower endogenous cysteine flux in the acutely malnourished stage but not in the mid-CUG stage, and they oxidized less cysteine at both stages resulting in better cysteine balance. There was no difference in SPU of cysteine between the edematous and nonedematous groups but ∼45% of enteral cysteine was extracted by the splanchnic bed in all children at both stages. These findings suggest that children with edematous SAM may have a greater requirement for cysteine during early and mid-nutritional rehabilitation because they used dietary cysteine more efficiently than did their nonedematous counterparts and that the splanchnic tissues of children with SAM have a relatively high requirement for cysteine.
Cysteine in the body's free amino acid pool derives from the diet, from de novo synthesis, and from the breakdown of body proteins. During early nutritional rehabilitation, endogenous cysteine flux, ie, cysteine derived from synthesis and protein breakdown, was slower in the edematous group than in the nonedematous group, which corroborated our previous finding (4). Despite similar dietary cysteine intakes by the 2 groups, oxidation rates were 58% and 67% slower in the edematous group than in the nonedematous group at stages 1 and 2, respectively, indicating a better efficiency of utilization of dietary cysteine and a higher demand for cysteine at both stages. A combination of higher cysteine consumption in synthetic pathways and lower contribution from protein turnover, especially in the acutely malnourished state, could have led to the lower oxidation rates and therefore lower concentrations in the edematous group. However, the edematous group could have maximized the availability of cysteine for synthetic purposes by lowering its rate of oxidation. This is supported by our comparison of the measured cysteine oxidation in the present study with the predicted obligatory cysteine oxidation using the approach of Young et al (17) (Table 4). To meet amino acid requirements, obligatory amino acid oxidation rates must be compensated for by an appropriate dietary supply that is greater than the obligatory loss (17, 18). Whereas cysteine oxidation relative to the predicted obligatory oxidation at stages 1 and 2 was 91% and 132%, respectively, in the nonedematous group, it was only 44% and 45%, respectively, in the edematous group. This further supports the notion of increased efficiency of utilization and higher cysteine requirements in the edematous group in response to insufficient supply relative to demands leading to an intrinsic minimum cysteine oxidation. Furthermore, the nonedematous children seemed to be oxidizing cysteine at the obligatory rate at stage 1, suggesting an increased demand when severely malnourished but to a much lesser extent than in the edematous group.
TABLE 4.
Predicted obligatory cysteine oxidation relative to measured cysteine oxidation1
| Protein flux2 | SAA/protein3 | SAA flux | SAA oxidation4 | Predicted cysteine oxidation5 | Measured cysteine oxidation | |
| g · kg · d−1 | mg/g | mg · kg · d−1 | mg · kg · d−1 | mg · kg · d−1 | mg · kg · d−1 | |
| Malnourished | ||||||
| Nonedematous | 7.8 | 35 | 275 | 27.5 | 16.5 | 15.1 |
| Edematous | 5.2 | 35 | 182 | 18.2 | 10.9 | 4.9 |
| Catch-up growth | ||||||
| Nonedematous | 9.8 | 35 | 343 | 34.3 | 20.6 | 27.3 |
| Edematous | 11.1 | 35 | 388 | 38.8 | 23.3 | 10.2 |
Predicted cysteine oxidation was calculated by using the approach of Young et al (17); measured oxidation values are from the present study. SAA, sulfur amino acid (cysteine and methionine).
Fasting state protein flux in early-childhood severe acute malnutrition (16), assuming that leucine content of 1 g body protein equals 75 mg (18), and during catch-up growth (19).
SAA composition of whole-body protein (18).
Assumes that endogenous amino acids are recycled with 90% efficiency (17).
Assumes that cysteine oxidation is 1.5 times that of methionine oxidation while receiving a sulfur amino acid–free diet (17).
The higher requirement for cysteine in the edematous children at stage 1 was not surprising because the need for early restoration of gut structure and function requires synthesis of cysteine-rich gastrointestinal proteins, mucins, and GSH. However, both groups had almost identical (∼5 μmol · kg−1 · h−1) cysteine SPU at stage 1, suggesting that the children with edema had a higher demand for cysteine to repair and replenish nonsplanchnic tissues. It was surprising to find that the greater need for cysteine persisted even at the mid-CUG stage in the edematous group when both groups were consuming as much as ∼20 μmol · kg−1 · h−1 dietary cysteine plus extra cysteine delivered as tracer (44.75 μmol/kg over 6 h). This extra amount of cysteine may have contributed to meeting overall demands and might have had a positive effect on GSH synthesis in the early phase of rehabilitation. Unfortunately, GSH kinetics was not measured.
There are several factors that could have contributed to the higher requirement for cysteine at the mid-CUG stage in the edematous group despite their large dietary cysteine intake. First, a marginally higher growth rate in the edematous group (19.5 g · kg−1 · d−1) compared with the nonedematous group (16.1 g · kg−1 · d−1) at this stage suggests greater cysteine utilization for body protein synthesis. Second, there will be an increased demand for cysteine to synthesize and replenish whole-body GSH; a previous study (1) showed that at the beginning of the CUG phase intracellular GSH concentration and synthesis are markedly reduced in children with edematous SAM. It is believed that increased consumption of GSH in edematous SAM (20, 21) for the disposal of free radicals generated from various noxae, especially infections, also contributes to lower GSH concentrations. This would contribute to an increased demand for cysteine to replenish GSH. However, in the present study there was no marked difference in the pattern of infections between edematous and nonedematous SAM, although the degree of infection was not clearly known. Third, 2 classical features of edematous SAM are thin, sparse hair and “flaky paint” dermatitis marked by erosion and ulceration of the skin. Both skin and hair contain cysteine-rich keratin, and biopsies of the skin of children with edematous SAM showed a large reduction in collagen, another cysteine-rich protein (22). From experience, we know that healing of the skin and restoration of normal hair continues well into the CUG period. Therefore, the need to synthesize skin and hair proteins may have also contributed to the higher demand for cysteine at the mid-CUG stage.
Severe atrophy of the intestinal mucosa and reduced mucins have been reported often in children with SAM (10, 23). Because mucosal and mucin proteins are rich in cysteine, it is expected that the demand for cysteine will be high to repair and restore gut function. This high demand is borne out by studies in piglets showing that intestinal epithelial cell proliferation is suppressed in sulfur amino acid–deficient piglets (11), and ∼80% of dietary cysteine is extracted and used by the portal drained viscera (24). On the basis of the report that gut mucosal atrophy was more pronounced in children with edematous SAM than in those with nonedematous SAM (10), we reasoned that this would contribute to greater SPU of cysteine in the children with edematous SAM, especially during the early phase of nutritional rehabilitation when gut integrity and function has to be reestablished to facilitate the increased dietary intakes needed for replenishment of body tissues. This was not the case because SPU was almost equally high in both groups. It is not certain whether or not intestinal uptake was different because SPU reflects uptake by the liver and portal drained viscera. However, at both stages SPU represented ∼45% of dietary cysteine, leaving only ∼55% to meet the requirements of the nonsplanchnic organs and tissue beds. In children with SAM this could have a negative effect on the overall synthesis of body proteins, especially those proteins rich in cysteine such as the skin and hair keratin (5). This may explain why the flaky-paint dermatitis lesions of children with edematous SAM take such a relatively long time (∼4 wk) to heal (5).
We previously reported that erythrocyte GSH concentration and synthesis rates are lower in edematous SAM than in nonedematous SAM (1), and that cysteine supplementation resulted in early restoration of erythrocyte GSH in children with edematous SAM (2). We also reported that cysteine released from protein breakdown was slower in children with edematous SAM (4). On the basis of these observations we argued that children with edematous SAM should be supplemented with cysteine, especially during the early stage of nutritional rehabilitation when they cannot tolerate a high protein intake (1, 2). The present data indicating greater requirement for cysteine at the level of the whole body are further support for a higher cysteine intake not only in the acutely malnourished phase but also during CUG.
Acknowledgments
We are grateful to the physicians and nursing staff of the Tropical Metabolism Research Unit for their care of the children and to Lorraine Wilson, O'Neil Brown, Bentley Chambers, Margaret Frazer, Melanie Del Rosario, and Vy Pham for their excellent work and support in the conduct of the studies and analysis of the samples.
The authors’ responsibilities were as follows—FJ, AB, MR and TF: designed the study and supervised various aspects of the study; CT-B: was responsible for clinical care of the subjects and participated in the isotope infusions; CG: prepared isotope infusates and processed samples: JWH: supervised laboratory analyses and calculated final data; and AB, FJ and JWH: analyzed and interpreted the data and wrote the manuscript. All authors contributed to different aspects of this study, including the design of the study, data collection, sample analysis, data interpretation, and writing of the manuscript. None of the authors had any conflicts of interest with the funding agencies.
Footnotes
Abbreviations used: CUG, catch-up growth; GSH, glutathione; IG, intragastric; IV, intravenous; SAM, severe acute malnutrition; SPU, splanchnic uptake.
REFERENCES
- 1.Reid M, Badaloo A, Forrester T, Morlese JF, Frazer M, Heird WC, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab 2000;278:E405–12 [DOI] [PubMed] [Google Scholar]
- 2.Badaloo A, Reid M, Forrester T, Heird WC, Jahoor F. Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am J Clin Nutr 2002;76:646–52 [DOI] [PubMed] [Google Scholar]
- 3.Jahoor F, Badaloo A, Reid M, Forrester T. Sulfur amino acid metabolism in children with severe childhood undernutrition: cysteine kinetics. Am J Clin Nutr 2006;84:1393–9 [DOI] [PubMed] [Google Scholar]
- 4.Jahoor F, Badaloo A, Reid M, Forrester T. Sulfur amino acid metabolism in children with severe childhood undernutrition: methionine kinetics. Am J Clin Nutr 2006;84:1400–5 [DOI] [PubMed] [Google Scholar]
- 5.Roediger WE. New views on the pathogenesis of kwashiorkor: methionine and other amino acids. J Pediatr Gastroenterol Nutr 1995;21:130–6 [DOI] [PubMed] [Google Scholar]
- 6.Neutra MR, Forstner JF. Gastrointestinal mucus: synthesis, secretion and function. : Johnson LR, Physiology of the gastrointestinal tract. 2nd ed New York, NY: Raven Press, 1987:474–975 [Google Scholar]
- 7.Dudley MA, Burrin DG, Wykes LJ, Toffolo G, Cobelli C, Nichols BL, Rosenberger J, Jahoor F, Reeds PJ. Parenteral nutrition selectively decreases protein synthesis in the small intestine. Am J Physiol 1998;274:G131–7 [DOI] [PubMed] [Google Scholar]
- 8.Faure M, Moënnoz D, Montigon F, Fay LB, Breuillé D, Finot PA, Ballèvre O, Boza J. Development of a rapid and convenient method to purify mucins and determine their in vivo synthesis rate in rats. Anal Biochem 2002;307:244–51 [DOI] [PubMed] [Google Scholar]
- 9.Bauchart-Thevret C, Stoll B, Burrin DG. Intestinal metabolism of sulfur amino acids. Nutr Res Rev 2009;22:175–87 [DOI] [PubMed] [Google Scholar]
- 10.Brunser O, Reid A, Monckeberg F, Maccioni A, Contreras I. Jejunal mucosa in infant malnutrition. Am J Clin Nutr 1968;21:976–83 [DOI] [PubMed] [Google Scholar]
- 11.Bauchart-Thevret C, Stoll B, Chacko S, Burrin DG. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab 2009;296:E1239–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mårtennson J, Jain A, Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci USA 1990;87:1715–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nkabyo YS, Gu LH, Jones DP, Ziegler TR. Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. J Nutr 2006;136:1242–8 [DOI] [PubMed] [Google Scholar]
- 14.Wellcome Working Party Classification of infantile malnutrition. Lancet 1970;2:302–3 [PubMed] [Google Scholar]
- 15.Jackson A, Golden M. Severe malnutrition. : Weatherall D, Ledingham J, Warrel D. Oxford textbook of medicine. Oxford, United Kingdom: Oxford University Press, 1988:12–3 [Google Scholar]
- 16.Jahoor F, Badaloo A, Reid M, Forrester T. Protein kinetic differences between children with edematous and nonedematous severe childhood undernutrition in the fed and postabsorptive states. Am J Clin Nutr 2005;82:792–800 [DOI] [PubMed] [Google Scholar]
- 17.Young VR, Bier DM, Pellett PL. A theoretical basis for increasing current estimates of the amino acid requirements in adult man, with experimental support. Am J Clin Nutr 1989;50:80–92 [DOI] [PubMed] [Google Scholar]
- 18.WHO Protein and amino acids requirements in human nutrition: report of a joint FAO/WHO/UNU expert consultation. World Health Organ Tech Rep Ser 2007;935:9–13 [PubMed] [Google Scholar]
- 19.Golden MH, Waterlow JC, Picou D. Protein turnover, synthesis and breakdown before and after recovery from protein-energy malnutrition. Clin Sci Mol Med 1977;53:473–7 [DOI] [PubMed] [Google Scholar]
- 20.Golden MH, Ramdath D. Free radicals in the pathogenesis of kwashiorkor. Proc Nutr Soc 1987;46:53–68 [DOI] [PubMed] [Google Scholar]
- 21.Jackson AA. Blood glutathione in severe malnutrition in childhood. Trans R Soc Trop Med Hyg 1986;80:911–3 [DOI] [PubMed] [Google Scholar]
- 22.Vasantha L. Labile collagen content in the skin in kwashiorkor. Clin Chim Acta 1969;26:277–80 [DOI] [PubMed] [Google Scholar]
- 23.Chandra J. Should malnourished children be included for defining normative values? Indian Pediatr 1991;28:1088–9 [PubMed] [Google Scholar]
- 24.Bos C, Stoll B, Fouillet H, Gaudichon C, Guan X, Grusak MA, Reeds PJ, Tomé D, Burrin DG. Intestinal lysine metabolism is driven by the enteral availability of dietary lysine in piglets fed a bolus meal. Am J Physiol Endocrinol Metab 2003;285:E1246–57 [DOI] [PubMed] [Google Scholar]