Abstract
BACKGROUND
The major sites of obstruction in chronic obstructive pulmonary disease (COPD) are small airways (<2 mm in diameter). We wanted to determine whether there was a relationship between small-airway obstruction and emphysematous destruction in COPD.
METHODS
We used multidetector computed tomography (CT) to compare the number of airways measuring 2.0 to 2.5 mm in 78 patients who had various stages of COPD, as judged by scoring on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) scale, in isolated lungs removed from patients with COPD who underwent lung transplantation, and in donor (control) lungs. MicroCT was used to measure the extent of emphysema (mean linear intercept), the number of terminal bronchioles per milliliter of lung volume, and the minimum diameters and cross-sectional areas of terminal bronchioles.
RESULTS
On multidetector CT, in samples from patients with COPD, as compared with control samples, the number of airways measuring 2.0 to 2.5 mm in diameter was reduced in patients with GOLD stage 1 disease (P = 0.001), GOLD stage 2 disease (P = 0.02), and GOLD stage 3 or 4 disease (P<0.001). MicroCT of isolated samples of lungs removed from patients with GOLD stage 4 disease showed a reduction of 81 to 99.7% in the total cross-sectional area of terminal bronchioles and a reduction of 72 to 89% in the number of terminal bronchioles (P<0.001). A comparison of the number of terminal bronchioles and dimensions at different levels of emphysematous destruction (i.e., an increasing value for the mean linear intercept) showed that the narrowing and loss of terminal bronchioles preceded emphysematous destruction in COPD (P<0.001).
CONCLUSIONS
These results show that narrowing and disappearance of small conducting airways before the onset of emphysematous destruction can explain the increased peripheral airway resistance reported in COPD. (Funded by the National Heart, Lung, and Blood Institute and others.)
Direct measurement of the distribution of resistance in the lower respiratory tract has established that small airways (i.e., <2 mm in internal diameter) become the major sites of obstruction in patients with chronic obstructive pulmonary disease (COPD).1–3 Resistance to flow through tubes is inversely related to the reduction in the radius raised to the fourth to fifth power. Since loss of half of such airways will only double the total peripheral resistance because of their parallel arrangement,4 the increase in peripheral airway resistance by a factor of 4 to 40, as has been reported in patients with COPD,1 is more easily explained by generalized narrowing than by loss of airways.
Diaz et al.5 used high-resolution computed tomography (CT) to show a reduced number of airways in regions of lung undergoing emphysematous destruction in patients with severe COPD. In this study, we examined the relationship between the numbers and dimensions of small airways and emphysematous destruction in patients with COPD. We used multidetector CT with a spatial resolution of 0.6 to 1.0 mm to count the number of airways measuring 2.0 to 2.5 mm and used microCT with a spatial resolution of 16.24 μm to measure the number and cross-sectional area of the much smaller terminal bronchioles. We also used histologic analysis to count the number of small airways per square centimeter and to measure the thickness of airway walls.
METHODS
PATIENTS AND LUNG SAMPLES
A total of 78 patients with COPD volunteered to undergo multidetector CT as part of a study of lung-cancer prevention6–8 (Table 1). We also collected data on 4 deceased patients who each donated a lung for transplantation, which served as a control lung when no suitable recipient was identified within the required time frame; 4 patients with centrilobular emphysema who each donated a lung; and 8 patients with panlobular emphysema who donated 10 lungs after lung transplantation (Table 2). Written informed consent was obtained from all patients and from the next of kin of the 4 patients whose donated lungs served as control samples.
Table 1.
Characteristics of the 78 Patients and Controls.*
| Characteristic | Controls (N = 20) | Patients with COPD | ||
|---|---|---|---|---|
| GOLD Stage 1 (N = 19) | GOLD Stage 2 (N = 19) | GOLD Stage 3 or 4 (N = 20) | ||
| Female:male ratio | 10:10 | 11:8 | 9:10 | 7:13 |
|
| ||||
| Age (yr) | 58.7±1.1 | 61.4±1.8 | 66.0±2.5 | 64.6±1.4 |
|
| ||||
| Height (cm) | 170.5±2.1 | 173.9±2.6 | 167.9±1.5 | 171.5±2.1 |
|
| ||||
| Weight (kg) | 82.9±3.8 | 78.0±3.8 | 79.3±2.5 | 79.3±3.7 |
|
| ||||
| Pack-yr of smoking (no.) | 43.3±2.7 | 45.3±2.4 | 49.9±5.0 | 54.6±3.8 |
|
| ||||
| FEV1 (% of predicted value) | 99.7±2.5 | 89.2±1.6 | 63.9±1.8 | 35.6±2.4 |
|
| ||||
| FEV1/FVC (%) | 78.6±1.0 | 65.2±0.9 | 62.2±1.5 | 46.2±2.5 |
|
| ||||
| Total lung volume (ml) | 4986±313 | 5884±340 | 5564±309 | 6747±432 |
|
| ||||
| Total lung mass (g) | 846±38 | 832±44 | 803±32 | 788±36 |
|
| ||||
| Total gas volume (ml) | 4192±284 | 5099±328 | 4810±288 | 6008±413 |
|
| ||||
| No. of airways measuring 2.0–2.5 mm in diameter | 177±10 | 129±9 | 136±13 | 54±9 |
Plus–minus values are means ±SE. There were no significant between-group differences except for age in the GOLD stage 2 group and the control group (P = 0.02). COPD denotes chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 second, FVC forced vital capacity, and GOLD Global Initiative for Chronic Obstructive Lung Disease.
Table 2.
Characteristics of 18 Isolated Lungs from Patients with Centrilobular or Panlobular Emphysema and Controls.*
| Characteristic | Controls (N = 4) | Patients with Centrilobular Emphysema (N = 4) | Patients with Panlobular Emphysema (N = 8) |
|---|---|---|---|
| No. of lungs | 4 | 4 | 10 |
| Female:male ratio | 0:4 | 2:2 | 3:5 |
| Age (yr) | 53.8±4.3 | 60.0±1.6 | 49.6±3.8 |
| Pack-yr of smoking (no.) | 31.5±7.5† | 43.0±5.5 | 17.9±3.2 |
| FEV1 (% of predicted value) | NA | 18.0±2.7 | 19.0±1.6 |
| FEV1/FVC (%) | NA | 26.8±2.9 | 32.6±2.3 |
| Total lung volume | |||
| % of predicted value | NA | 137.0±3.6 | 140.1±4.1 |
| Volume (ml) | 3251±261 | 3456±602 | 3794±595 |
| Lung mass (g) | 332±11 | 358±27 | 394±41 |
| Terminal bronchioles | |||
| No./ml of lung volume | 6.9±1.2 | 0.7±0.1 | 1.6±0.5 |
| Total no. | 22,300±3900 | 2400±600 | 6200±2100‡ |
| Cross-sectional area of terminal bronchioles (mm2) | |||
| Average | 0.145±0.025 | 0.004±0.002 | 0.047±0.012 |
| Total | 3050.3±576.6 | 7.7±5.1 | 514.1±181.9‡ |
| Minimum luminal diameter (μm) | 424.0±48.0 | 51.8±30.0 | 210.2±48.0 |
Plus–minus values are means ±SE. FEV1 denotes forced expiratory volume in 1 second, FVC forced vital capacity, and NA not available.
The number of pack-years was measured in two controls, since the other two controls were nonsmokers.
The total number was measured in seven patients.
STUDY DESIGN
We assessed the airways at two levels of resolution. We measured the number of small airways (diameter, 2.0 to 2.5 mm) per lung as seen on thoracic multidetector CT in the 78 patients who had varying degrees of severity of COPD, in the 4 control lungs, and in the 14 lungs from 12 patients who were undergoing lung transplantation for stage 4 COPD, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging system (with stage 4 indicating the most severe disease). In 175 samples of lung tissue removed from these 18 isolated lungs, we used microCT to measure the mean linear intercept (an average alveolar dimension), the number of terminal bronchioles (the last bronchioles without alveolar openings from their walls) per milliliter of lung volume, and the diameters and cross-sectional areas of terminal bronchioles. The total lung volume, which was determined from measurements on multidetector CT, served as the reference volume to compute both the total number of terminal bronchioles and total cross-sectional area of terminal bronchioles per lung. These values were doubled to obtain values per lung pair.
MULTIDETECTOR CT
Each of the 78 patients from the study of lung-cancer prevention underwent volumetric multi-detector CT at full inspiration. Scans were obtained in the volume-scan mode of a Siemens Sensation 16 scanner at 120 kVp, 125 mAs, and 1.0-mm slice thickness, with the use of B35f and B60f reconstruction filters. We used these scans to compute total lung, gas, and tissue volumes, and the Disector method (which uses a pair of serial sections separated by a known distance) was used to count the number of visible small airways per milliliter of lung volume (Section 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Briefly, a reference volume frame was provided by 30 pairs of images of 1 mm in thickness that were separated by a 2-mm distance and that were evenly spaced between the lung apex and base. The measured mean number of airways per milliliter of lung volume was multiplied by the total lung volume, as measured on multidetector CT, to calculate the total number of airways with a diameter of 2.0 to 2.5 mm per lung pair.
The main-stem bronchus of each of the 18 isolated lungs was cannulated9 and attached to a source of compressed air with an underwater seal that allowed the lungs to be gently inflated to a transpulmonary pressure of 30 cm of water, deflated to a transpulmonary pressure of 10 cm of water, and frozen solid in liquid nitrogen vapor at −130°C. Each specimen was kept frozen in a Styrofoam box containing dry ice while volumetric multidetector CT was performed (according to the protocol described for thoracic multidetector CT) and then stored at −80°C. These multidetector CT scans were used to systematically follow each pathway from the main-stem bronchus to the last visible bifurcation (the point at which one airway branches into two or more smaller airways), and the number of branches at each generation was recorded (Section 2 in the Supplementary Appendix).
MICROCOMPUTED TOMOGRAPHY
The frozen lung specimens were maintained on dry ice at −78.2°C while they were cut into slices with a thickness of 2 cm in the same plane as that used for the multidetector CT scan. Samples were removed in clusters with the use of a sharpened cylinder measuring 14 mm in diameter to cut the cores of lung tissue processed for microCT and a 16-mm cylinder to cut three companion cores of tissue adjacent to each sample removed for microCT. All the samples were stored at −80°C, and their position was recorded on the multi-detector CT scan for the corresponding specimen by matching before-and-after photographs of the slices to the appropriate multidetector CT slice image (Fig. 1). The representative nature of these samples with respect to the entire lung was established by comparing the densities of the sampled sites with the frequency distribution of the densities in the entire lung on multidetector CT (Section 3 in the Supplementary Appendix).
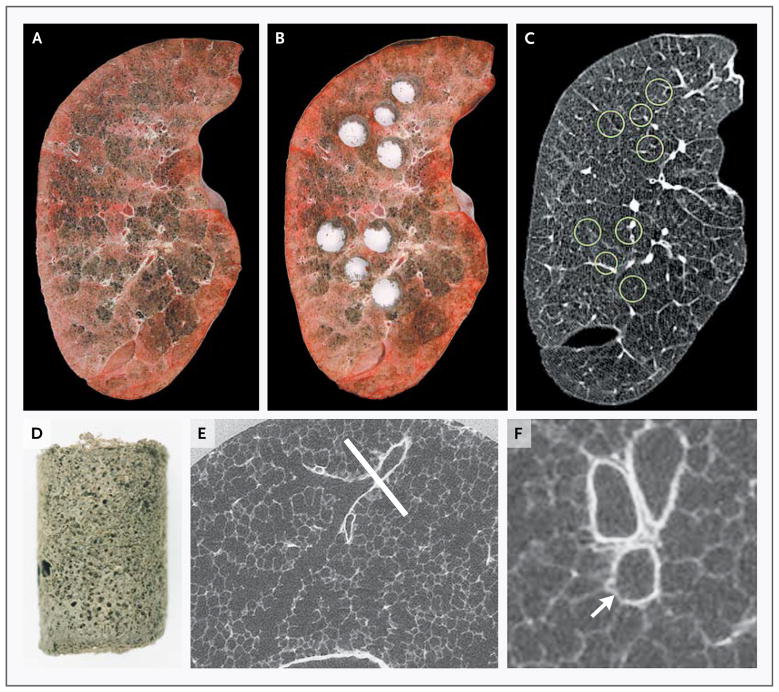
Figure 1. Lung-Tissue Samples Matched with CT Images.
Panel A shows a frozen lung slice from a patient with severe centrilobular emphysema, and Panel B shows the same lung slice after samples were removed for analysis. Panel C shows the matching slice from the multidetector CT scan of the intact lung specimen, with the location of samples indicated by circles. Panel D shows a single control lung sample after it was processed for microCT. Panel E shows a microCT image of a control lung at a resolution of 16.24 μm, with a terminal bronchiole (indicated by the white line) at the point at which it branches into respiratory bronchioles. Panel F shows the same terminal bronchiole reoriented to show the cross section of the airway (arrow) at the plane of the section indicated by the line in Panel E.
The 175 cores of tissue that were processed for microCT were fixed at −80°C in a 1% solution of glutaraldehyde in pure acetone (freezing point, −94.7°C), warmed to room temperature overnight, washed in acetone, fixed in 1% osmium tetroxide in acetone, rewashed in ethanol, and dried with a critical-point procedure (Autosamdri-815B, Tousimis). The specimens that were prepared for microCT were scanned with the use of either an eXplore Locus SP MicroCT scanner (GE Health-care) at the University of Pennsylvania or a Scanco MicroCT 35 scanner (Scanco Medical) at the University of British Columbia (Fig. 1D). The protocol for microCT provided 16.24-μm isotropic voxel resolution and 460 to 1000 contiguous microCT images per tissue core, with an x-ray tube peak voltage of 80 kVp and a current of 80 μA, 3 seconds of exposure time, 500 views at 0.4-degree increments (short scan), 1 × 1 pixel binning, and 4 averages.
MicroCT scans were examined in contiguous sections, and terminal bronchioles were identified by following small conducting airways to the point at which they branched into respiratory bronchioles (Fig. 1E, and Section 4 in the Supplementary Appendix). The number of terminal bronchioles per milliliter of lung volume was recorded, and five randomly selected terminal bronchioles from each lung were examined with the use of multiplanar reconstruction software (OsiriX 2.7.5, OsiriX Foundation) to reorient images in three dimensions and measure their diameter and luminal cross-sectional area at the narrowest point (Fig. 1F). The product of the mean number of terminal bronchioles per milliliter of each lung, as measured on microCT, and the total lung volume, as measured on multidetector CT of the same lung, provided an estimate of the total number of terminal bronchioles per lung or lung pair. The product of the total number of terminal bronchioles per lung and the average cross-sectional area provided the total cross-sectional area of all terminal bronchioles in each lung. The mean linear intercept, which has a direct linear relationship with air-space size,10,11 was measured from images captured at 20 regularly spaced intervals within the microCT scans of each sample with the use of a previously validated grid of test lines projected onto the image and a custom macro (Image Pro Plus, Media Cybernetics).
HISTOLOGIC ANALYSIS
Portions of tissue from 74 lung-tissue cores that were examined on microCT were embedded in JB4 plastic, and sections with a thickness of 4 μm were cut and then stained with toluidine blue. We measured the mean linear intercept on these histologic sections using the same protocol that was used for the microCT images (Section 5 in the Supplementary Appendix). We used the Disector method to examine 8 of 74 of the JB4-embedded blocks.12–14 Sections that were 4 μm thick and 720 μm apart were used to define a volume frame of 0.072 ml. We counted the number of bronchioles per milliliter and compared them with the number per milliliter as determined on microCT in the same frame (Section 6 in the Supplementary Appendix). Portions of 64 companion cores (measuring 16 mm in diameter) that were cut adjacent to those examined by microCT were vacuum-embedded in solution with 50% vol/vol Tissue-Tek O.C.T. compound (Sakura Finetek) in phosphate-buffered saline and 10% sucrose at 1°C and immediately refrozen on dry ice. Cryosections that were cut from the frozen tissue blocks were used to count bronchiolar profiles per square centimeter and measure bronchiolar diameter and wall thickness, as described previously.15
STATISTICAL ANALYSIS
The numbers of airways that were counted on multidetector CT and histologic data for patients according to GOLD stage were compared with the use of Tukey’s method of pairwise comparison. We used the Mann–Whitney test to compare the number of airways at each generation of branching, as measured on CT, and Student’s t-test to compare the number of terminal bronchioles. Data are expressed as means ±SE.
RESULTS
MULTIDETECTOR CT
Table 1 summarizes the data regarding demographic characteristics, lung function, and total lung, tissue, and gas volumes for all 78 patients who participated in this part of the study. As compared with airways in control samples, the number of airways measuring 2.0 to 2.5 mm in diameter per lung pair was reduced in patients with GOLD stage 1 disease (P = 0.001) and GOLD stage 2 disease (P = 0.02) and was further reduced in patients with GOLD stage 3 or 4 disease (P<0.001) (Fig. 2A). Table 2 summarizes the demographic data for the 16 patients who donated 18 lung specimens that were examined on volumetric multi-detector CT. We compared the distribution of airways identified on multidetector CT reconstructions of the bronchial tree from scans of the control lungs with published data on the distribution of airways measuring 2.0 mm, 2.5 mm, 3.0 mm, and 4.0 mm in diameter from airway casts16 (Fig. 2B). Although the results showed that this procedure identified most of the 2.5-mm airways but only some of the 2-mm airways previously reported from casts, it also showed that in comparison with airways in the control lungs, the number of airways measuring 2.0 to 2.5 mm was reduced in isolated lungs from 4 patients with centrilobular phenotypes and 4 patients with panlobular phenotypes of GOLD stage 4 disease.
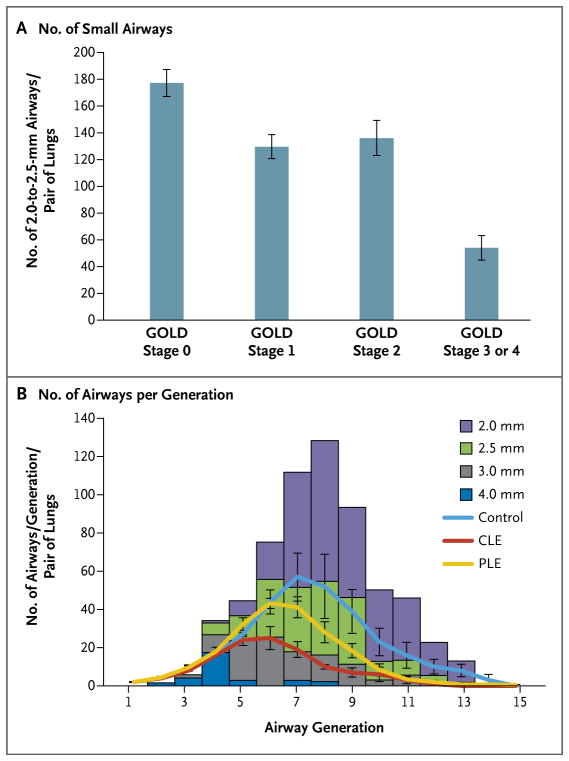
Figure 2. Numbers of Small Airways and Airways per Generation of Branching, According to the Severity of COPD.
Panel A shows the number of airways measuring 2.0 to 2.5 mm in diameter per lung pair that were obtained with the use of a computed tomographic (CT) Disector method to analyze the multidetector CT scans from 78 patients who had various stages of COPD. As compared with the control group, there was a reduced number of small airways per lung pair in patients with stage 1 disease on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) scale (P = 0.001), GOLD stage 2 disease (P = 0.02), and GOLD stage 3 or 4 disease (P<0.001). Panel B shows data obtained by reconstructing the bronchial tree from multidetector CT images of explanted lung specimens. The height of the columns represents the number of airways in each generation (the point at which one airway branches into two or more smaller airways), colored according to the size that was previously reported from lung casts.16 The number of airways per generation that were obtained in this study shows that control lungs closely match the distribution of airways down to and including those measuring 2.5 mm in diameter. In contrast, in lungs from four patients with centrilobular emphysema (CLE), the number of airways at each generation is lower than predicted, and airways measuring 2.5 mm in diameter or less are largely missing or narrowed to the point of being below the resolution of the multidetector CT scan, with lungs from four patients with panlobular emphysema (PLE) falling between values for those with CLE and for four control lungs. The I bars indicate standard errors.
MICROCT AND HISTOLOGIC FINDINGS
Control lungs contained 6.9±1.2 terminal bronchioles per milliliter of lung volume, with an average diameter of 424±38 μm and a cross-sectional area of 0.145±0.036 mm2 (Table 2). The total number of terminal bronchioles was 22,300±3900, and the total cross-sectional area of terminal bronchioles was 3050.3±576.6 mm2 per lung. In comparison, lungs from patients with the centrilobular emphysematous phenotype of COPD had a reduction of 99.7% in the terminal bronchiolar cross-sectional area per lung and a reduction of 89% in the total number of terminal bronchioles per lung (P<0.001 for both comparisons). Moreover, explanted lungs from patients with the panlobular emphysema phenotype had a reduction of 83% in the total cross-sectional area and a reduction of 72% in the number of terminal bronchioles (P<0.001 for both comparisons).
Measurements of the mean linear intercept that were made at regular intervals from the lung apex to the base varied little in control lungs and, as expected, increased in the upper regions of lungs affected by centrilobular emphysema (Fig. 3A) and in the middle and lower regions of lungs affected by panlobular emphysema (Fig. 3B). An increasing value for the mean linear intercept, as compared with that in control lungs, was observed in both the centrilobular and panlobular phenotypes of COPD (Fig. 3C). There was a sharp reduction in the number of terminal bronchioles per milliliter of lung volume in regions of diseased lungs in the centrilobular emphysematous phenotype of COPD in regions in which the mean linear intercept remained below the upper limit of the 95% confidence interval (<489 μm) for the control lungs (P<0.001) (Fig. 3D). There also was a sharp reduction in the number of airway profiles per square centimeter in regions of the diseased lungs affected by centrilobular emphysema in which the mean linear intercept remained below the 489-μm limit observed in the control lungs (P = 0.002) (Fig. 4A). The remaining airways had thickened airway walls in the lungs affected by centrilobular emphysema, as compared with controls (P<0.001) (Fig. 4B). Data on interobserver and intraobserver agreement for measurements on both multidetector CT and microCT are provided in Section 7 in the Supplementary Appendix.
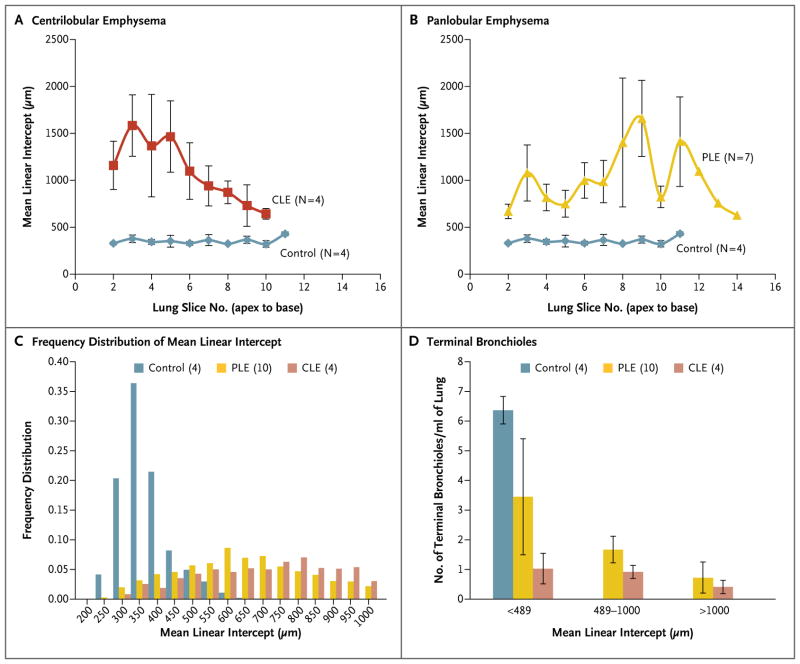
Figure 3. Mean Linear Intercept and Number of Terminal Bronchioles, According to the Emphysematous Phenotype of COPD.
Measurements of the mean linear intercept show the expected distribution of emphysema from lung apex to base in lungs from 4 patients with centrilobular emphysema (CLE) (Panel A) and 7 patients with panlobular emphysema (PLE) (Panel B), with no change as a function of lung-slice number in the 4 control lungs. In Panel C, the frequency distribution of measurements of the mean linear intercept is shown in the 4 control lungs, as compared with the frequency distribution in the 4 lungs affected by CLE and 10 lungs affected by PLE. In Panel D, the regions of the diseased lungs in which the mean linear intercept remained below the upper limit of the 95% confidence interval for the control lungs (<489 μm) have a reduced number of terminal bronchioles per milliliter of lung volume in the CLE group (P<0.001) and remain low in samples with a mean linear intercept of 489 to 1000 μm and of more than 1000 μm. The I bars indicate standard errors.
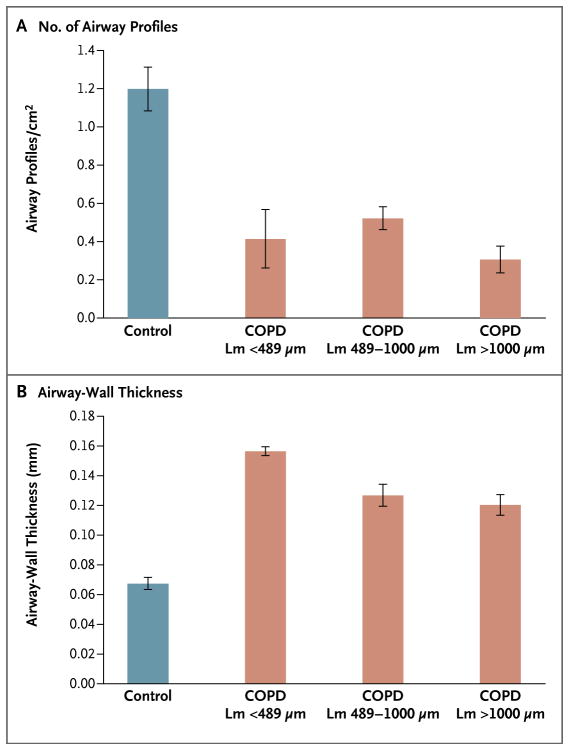
Figure 4. Airway Profiles and Airway-Wall Thickness, According to the Extent of Emphysema in COPD.
Shown are the number of small-airway profiles per square centimeter (Panel A) and the thickness of the airway walls (Panel B), as measured from histologic sections cut from samples of tissue adjacent to those examined on microCT. The number of small-airway profiles per unit area is sharply reduced in regions of diseased lungs in which the mean linear intercept (Lm) remains below the 95% confidence interval (489 μm) observed in control lungs and the surviving airways have thickened walls. The I bars indicate standard errors.
DISCUSSION
Our data show that the number of small airways (2.0 to 2.5 mm in diameter) per lung pair was reduced in patients with mild COPD (GOLD stage 1), as compared with control samples, and was further reduced in patients with severe or very severe COPD (GOLD stage 3 or 4). Although a comparison of the number of airways present at each generation of branching that was observed on multi-detector CT of the isolated control lungs did not identify all the 2-mm airways reported from airway casts of normal lungs,16 the comparison of the results for control samples and those for diseased lungs showed fewer airways measuring 2.0 to 2.5 mm in both centrilobular and panlobular emphysematous phenotypes of COPD.
However, we could not determine whether the reduction in the number of small airways that was observed by either method of multidetector CT analysis was a true reduction in number or simply a narrowing to the point at which the airways were no longer visible at a spatial resolution of approximately 1 mm. In contrast to the multidetector CT scans, the 16.24-μm spatial resolution of the microCT scans of the 4 control lungs provided mean terminal bronchiolar diameters and cross-sectional areas that were consistent with published normal values (Section 8 in the Supplementary Appendix). Furthermore, our comparison of the 4 control lungs with the 14 lungs from patients with very severe COPD (GOLD stage 4) clearly showed that the total number of terminal bronchioles and total cross-sectional areas were substantially reduced in both the centrilobular and panlobular emphysematous phenotypes of COPD. A comparison of microCT measurements of the number of terminal bronchioles per milliliter of lung volume with the alveolar dimensions (mean linear intercept) that were measured within the same lung samples showed that narrowing and loss of terminal bronchioles clearly preceded the appearance of microscopical emphysematous destruction in the centrilobular emphysematous phenotype of COPD. Although a similar trend was present in the panlobular emphysematous phenotype, this trend did not become significant until the mean linear intercept increased beyond the control range (>489 μm) (Fig. 3D, and Section 9 in the Supplementary Appendix). These results extend Leopold and Gough’s17 classic description of centrilobular emphysema (suggesting that these lesions start in terminal bronchioles) by showing that the terminal bronchioles were narrowed and destroyed before the onset of emphysematous destruction.
Bignon et al.18 were the first investigators to measure small-airway narrowing in COPD by showing that the number of bronchiolar profiles with a luminal diameter of less than 400 μm increased on postmortem analysis of lungs obtained from patients who had died from respiratory failure. Although Matsuba and Thurlbeck19 confirmed the finding of Bignon et al., they concluded that this change was too small to account for the increase by a factor of 4 to 40 in the peripheral resistance reported in COPD (Section 10 in the Supplementary Appendix). However, they added the caveat that the disappearance of large numbers of the smallest bronchioles might have buffered the downward shift of mean bronchiolar diameter that they observed in diseased lungs.
In our study, the data on airway profiles per square centimeter are similar to those reported by both Bignon et al. and Matsuba and Thurlbeck (Fig. 4A, and Section 10 in the Supplementary Appendix). However, it is not possible to compute the total number of terminal bronchioles per milliliter of lung volume from profiles per unit of area without following the principles of stereology and defining a volume in which the profiles are measured. In contrast, the use of microCT allowed for the precise identification of terminal bronchioles (videos 1 and 2, available at NEJM.org, and Section 4 in the Supplementary Appendix) and counted in known volumes of lung tissue. Moreover, the multiplanar-reconstruction software that was used to analyze these microCT images allowed for the examination of individual terminal bronchioles in multiple planes to accurately measure their luminal cross-sectional area at its narrowest point. The product of the mean value of terminal bronchioles per milliliter of lung volume, as computed on microCT, and total lung volume, as computed on multidetector CT, provided an estimate of the total number of terminal bronchioles per lung.
Major limitations of microCT are that the required radiation dose can be safely applied only to samples of isolated lungs and that the cost of the procedure limits its application to a relatively small number of specimens. Our sampling design made it impossible to determine whether airways measuring 2.0 to 2.5 mm in diameter actually disappeared or simply narrowed to the point at which they were no longer visible on multi-detector CT. Despite these limitations, the microCT results extend earlier reports by showing that there is both widespread narrowing and loss of smaller conducting airways before the onset of emphysematous destruction in both centrilobular and panlobular emphysema phenotypes of COPD. This process readily explains the observed increase by a factor of 4 to 40 in small-airway resistance in patients with COPD.
Supplementary Material
Acknowledgments
Supported by grants (HL084922, HL084948, HL062194, and HL090806) from the National Heart, Lung, and Blood Institute, by the Canadian Institute of Health Research–Thoracic Imaging Network of Canada, by the Canadian Collaborative Innovative Research Fund, by GlaxoSmithKline, and by the Lavin Family Supporting Foundation.
We thank Nerissa Tai, Crystal Leung, Ricky Lo, and Irina Vicol for their technical help with the manuscript; the late Dr. Peter Macklem provided helpful comments in the early stages of this study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–60. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 2.Van Brabandt H, Cauberghs M, Verbeken E, Moerman P, Lauweryns JM, Van de Woestijne KP. Partitioning of pulmonary impedance in excised human and canine lungs. J Appl Physiol. 1983;55:1733–42. doi: 10.1152/jappl.1983.55.6.1733. [DOI] [PubMed] [Google Scholar]
- 3.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol. 1992;72:1016–23. doi: 10.1152/jappl.1992.72.3.1016. [DOI] [PubMed] [Google Scholar]
- 4.Pedley TJ, Schroter RC, Sudlow MF. The prediction of pressure drop and variation of resistance within the human bronchial airways. Respir Physiol. 1970;9:387–405. doi: 10.1016/0034-5687(70)90094-0. [DOI] [PubMed] [Google Scholar]
- 5.Diaz AA, Valim C, Yamashiro T, et al. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest. 2010;138:880–7. doi: 10.1378/chest.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam S, MacAulay C, Le Riche JC, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J Natl Cancer Inst. 2002;94:1001–9. doi: 10.1093/jnci/94.13.1001. [DOI] [PubMed] [Google Scholar]
- 7.Lam S, leRiche JC, McWilliams A, et al. A randomized phase IIb trial of Pulmicort Turbuhaler (budesonide) in people with dysplasia of the bronchial epithelium. Clin Cancer Res. 2004;10:6502–11. doi: 10.1158/1078-0432.CCR-04-0686. [DOI] [PubMed] [Google Scholar]
- 8.McWilliams AM, Mayo JR, Ahn MI, MacDonald SL, Lam SC. Lung cancer screening using multi-slice thin-section computed tomography and autofluorescence bronchoscopy. J Thorac Oncol. 2006;1:61–8. [PubMed] [Google Scholar]
- 9.Choong CK, Haddad FJ, Martinez C, et al. A simple, reproducible, and inexpensive technique in the preparation of explanted emphysematous lungs for ex vivo studies. J Thorac Cardiovasc Surg. 2005;130:922–3. doi: 10.1016/j.jtcvs.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Dunnill MS. Quantitative methods in the study of pulmonary pathology. Thorax. 1962;17:320–8. doi: 10.1136/thx.17.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbesom AA, Versteeg EM, Veerkamp JH, et al. Morphological quantification of emphysema in small human lung specimens: comparison of methods and relation with clinical data. Mod Pathol. 2003;16:1–7. doi: 10.1097/01.MP.0000043519.29370.C2. [DOI] [PubMed] [Google Scholar]
- 12.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–36. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 13.Howard CV, Reed MG. Unbiased stereology: three-dimensional measurement in microscopy. Oxford, England: BIOS Scientific; 1998. [Google Scholar]
- 14.Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 16.Weibel ER. Morphometry of the human lung. New York: Academic Press; 1963. [Google Scholar]
- 17.Leopold JG, Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax. 1957;12:219–35. doi: 10.1136/thx.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bignon J, Khoury F, Even P, Andre J, Brouet G. Morphometric study in chronic obstructive bronchopulmonary disease: pathologic, clinical, and physiologic correlations. Am Rev Respir Dis. 1969;99:669–95. doi: 10.1164/arrd.1969.99.5.669. [DOI] [PubMed] [Google Scholar]
- 19.Matsuba K, Thurlbeck WM. The number and dimensions of small airways in emphysematous lungs. Am J Pathol. 1972;67:265–75. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.