Abstract
Elevated Tricuspid Regurgitant Velocity (TRV) has been related to higher mortality in adults and to hemolysis, lower oxygen saturation during 6-minute walk test and acute chest syndrome (ACS) in children with sickle cell disease (SCD). Hydroxyurea (HU) has reduced TRV value in children and adults. We describe a three year old HbSS child with recurrent ACS, hypoperfusion of the left lung, mild hemolysis and persistent TRV elevation. TRV did not normalize after HU, despite improvement in clinical conditions and in baseline laboratory parameters related to hemolysis and blood viscosity, but normalized after bone marrow transplantation (BMT). Our experience suggests that in young patients, TRV reduction can be a positive concomitant effect of BMT.
Key words: sickle-cell disease; pulmonary hypertension; tricuspid regurgitant jet velocity; hydroxyurea, bone marrow transplantation.
Introduction
Tricuspid Regurgitant Velocity (TRV) has become a reliable marker to screen for doppler-defined pulmonary hypertension (PH) in sickle cell disease (SCD).1 Elevated TRV has been related to higher mortality in adults2 and to hemolysis, lower oxygen saturation during 6-minute walk test3 and acute chest syndrome (ACS)4 in children, even though it's clinical relevance, especially in the paediatric age, is not yet clearly defined. It remains to be determined whether TRV, and its association with mortality, reflects true PH or is a biomarker of disease severity and systemic vasculopathy in SCD.5 In fact, even if TRV measurement on echocardiography can be a first tool to screen for PH in patients with SCD, recent studies have clearly shown that only a limited number of patients with TRV elevation have PH as confirmed on cardiac catheterization6 and that different factors could play a role in leading to TRV elevation in different subsets of patients.7–8 Moreover, the causes involved in the genesis of elevated TRV (role of hemolysis and vasoocclusive thromboembolic factors) and of PH in SCD are still under investigation.5,9–10
Clinical management of TRV elevation is also not clear even if Hydroxyurea (HU) has been demonstrated to lower TRV value in children and adults.11–12 The effect of bone marrow transplantation (BMT) on TRV value and Doppler-defined PH in SCD has not yet been reported. We describe a three year old HbSS child with recurrent ACS -resulting in lung hypoperfusion-, mild hemolysis and TRV elevation since age two. He did not improve after HU treatment but 1 year after undergoing BMT from an HbAA HLA-related sibling, presents no signs of hemolysis and normal TRV, although lung damage remained.
Case Report
A 21-months Nigerian male was diagnosed with HbSS in occasion of the first ACS presenting with infiltration in the left lung and pleural effusion. At 23 and 26 months he experienced a second and third ACS with infiltration of the left lobe. Steady state hematological parameters are shown in Table 1, while steady state Blood Pressure (BP) and SatO2 were above the 90th percentile and 97–98%, respectively. Steady state TRV at 28 months was 2.8 m/sec.
Table 1. Laboratory values before HU treatment, after 1 year of HU treatment and 1 year after bone marrow transplantation.
| Before HU | 1 year After HU | 1 year After BMT | ||
|---|---|---|---|---|
| Variable | Mean±SD | Mean±SD | Mean±SD | P |
| Aspartate aminotransferase (U/L) | 80.67±21.03 | 53.88±7.02 | 57.25±13.52 | 0.021 |
| Indirect bilirubin (mcmol/L) | 49.95±7.28 | 25.67±2.65 | 5.10±1.41 | 0.000 |
| White Blood Cells (×109/L) | 15840±4343.15 | 9704.17±2245.44 | 4745.83±1739.31 | 0.000 |
| Hemoglobin (g/dL) | 7.08±0.46 | 8.10±0.49 | 12.29±0.64 | 0.000 |
| Hb F (%) | 5.10±0.62 | 17.31±1.96 | 2.15±2.76 | 0.000 |
| Platelets (×109/L) | 550600±228802.97 | 393769.23±170706.94 | 270090.91±34725.94 | 0.006 |
| Reticulocytes (×109/L) | 422500±32809.75 | 345016.67±113780.79 | 18300±10137.06 | 0.001 |
At 30 months he began HU 10 mg/kg, gradually increased to 30 mg/kg in four months. The treatment was well tolerated.
At 42 months, 1 year after starting HU, despite improvement of hematological parameters, TRV was still 2.82 m/sec. BP had dropped to normal values. ECG and cardiac ultrasound (including Tissue Doppler) at 21, 38 and 43 months were normal. He never presented with obstructive sleep apnea or asthma. Transcranial Doppler (TCD) velocities remained conditional before HU and after HU -time averaged mean velocity of maximum blood flow (TAMM) of 185 cm/sec and 189 cm/sec, respectively.
Having experienced recurrent ACS and persistent high conditional velocities on TCD, due to availability of an HbAA HLA- matched sibling he was offered BMT.
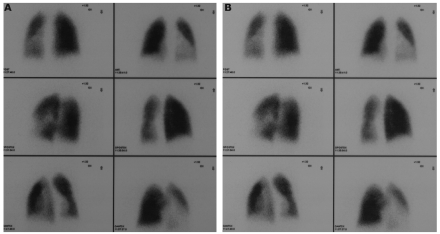
As part of the BMT work-up he underwent lung CT scan showing numerous perihilar strie dense in the lower left lobe, with increased density of the parenchyma, interpreted as lung scars due to the recurrent ACS, and pulmonary perfusion scintigraphy revealing hypoperfusion of the entire left lung, mainly of the left lower lobe respectively (Figure 1).
Figure 1.
Pulmonary perfusion scintigraphy before (A) and 1 year after BMT (B)
At 47 months he received BMT with the following conditioning regimen: Thiotepa 8 mg/kg/day (70 mg ×2 times on day −7), Treosulfan 14 g/m2/day (9.8 gr on day −6, −5 and −4), Fludarabine 40 mg/m2/day (28 mg on day −6,−5,−4 e −3), antithymocyte globulin 175 mg/day (on day −4,−3 and −2). Polymorphonucleated leukocytes and platelet engraftment occurred at day +20 and +22 respectively. No transplant related complications were observed.
One year after BMT he presents with 100% chimerism, no HbS on electrophoresis and is currently well, having experienced no SCD-related complications. Hematological and biochemical values are within normal range for age (Table 1) and TRV dropped to 2.01 m/sec. A pulmonary perfusion scintigraphy performed 1 year after BMT still shows persistence of hypoperfusion of the left lung (unchanged from pre-BMT perfusion scintigraphy).
Discussion
Our case represents, to our knowledge, the first reported case of a very young SCD child with TRV elevation that did not reduce after HU treatment, but normalized after BMT.
Several factors contribute to the increase in TRV in SCD children, including increased pulmonary flow volume (cardiac output), increased left ventricular filling pressures, increased blood viscosity, and increased pulmonary vascular resistance.8 SCD children can also experience systemic hypertension, left-sided volume overload, and abnormal diastolic function, all of which lead to elevated left ventricular filling pressures and secondary elevation of pulmonary artery pressures. Our patient had surely several of the above mentioned factors.
The lack of response of TRV values to HU has been described in only one older patient with severe PH11 and HU efficacy in reducing TRV values is still open. In fact, while some studies demonstrated the protective effect of HU on the development of PH in adults12 and children,11 others have not and HU-induced high haemoglobin F levels have, on the contrary, been related to higher TRV velocities.13 HU has reversed Doppler-defined PH in SCD children11 and adults12 probably due to the combined reduction of hemolysis and red cell adhesion, and to the improvement of vascular function,14 even when the increase in haemoglobin concentration was modest (from 8.3 to 8.7 g/dL and from 7.52 to 7.98 g/dL).11,12 But HU was not effective in reducing TRV in our patient, despite the increase in Hb level (from 7.08 to 8.1 g/dL), the significant increase of HbF% and the reduction of hematologic parameters related to hemolysis (reticulocytes, AST) and to blood viscosity (platelets, white blood cells). Additive effect during HU treatment was normalization of the BP to the 50th percentile, while TCD velocities remained conditional (TAMM of 185 cm/sec, 189 cm/sec and 105 cm/sec before HU, after HU and after BMT respectively).
One year after BMT, all haematological parameters and TRV gradually returned to normal values (2.7, 2.5, 2 m/sec at 3, 8 and 12 months after BMT) while pulmonary scintigraphy remained impaired, suggesting a non causative role of in situ thrombosis in generating high TRV in our patient. In fact, in situ thrombosis does occur in PH, but seems to represent a primary aetiology only in a limited number of cases.15
BMT has reversed both hemolysis and PH in two cases of Evans Syndrome presenting with severe clinical manifestations.16 BMT has also substantially reduced cranial blood velocity17 and succeeded in stabilizing both cerebral vasculopathy and lung function in paediatric patients with SCD.18,19
The normalization of TRV in our patient after BMT, despite the persistent hypoperfusion of the left lung, might be due to the HbAA BMT-related elimination of hemolysis and inflammation, with a substantial increase in haemoglobin (from 8.01 to 12.3 g/dL), and to the improvement of vascular function. In fact, while HU reduces hemolysis, reduces adhesion molecules (on red cells, platelets, WBC and vascular endothelium) and seems to improve vascular tone14 while displaying controversial effects on inflammation,20 BMT-especially if a BMT with HbAA donor- eliminates hemolysis and inflammation, improves rheological characteristics and determines substantial vascular repair with restoration of the endothelial lining and maintenance of vascular homeostatis.21
Under the umbrella of Doppler-defined PH probably fall different subgroup of patients in which the various pathogenetic events (hemolysis, oxidant stress, inflammatory stress, chronic thromboembolism, in situ thrombosis, chronic hypoxemia with activation of proliferative mediators, parenchymal and vascular injury) may be differently involved and may require different therapeutic approaches.9,10,22 In very young patients, TRV elevation might express a biomarker of disease severity and systemic vasculopathy5 instead of true PH and only prospective trials evaluating pulmonary vascular resistance could aid in clarifying this issue.
Nevertheless, our case shows that TRV reduction in young patients, having experienced recurrent ACS with mild hemolysis and persistent TRV elevation resistant to HU treatment, can be a positive concomitant effect of BMT.
Acknowledgements:
the research was performed with grant 07/04 from the Fondazione Citta` della Speranza.
References
- 1.Ambrusko SJ, Gunawardena S, Sakara A, et al. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatric Blood and Cancer. 2006;47:907–13. doi: 10.1002/pbc.20791. [DOI] [PubMed] [Google Scholar]
- 2.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 3.Minniti CP, Sable C, Campbell A, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94:340–7. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombatti R, Maschietto N, Varotto E, et al. Pulmonary hypertension in sickle cell disease children under 10 years of age. Br J Haematol. 2010;150:601–9. doi: 10.1111/j.1365-2141.2010.08269.x. [DOI] [PubMed] [Google Scholar]
- 5.Morris CR. Vascular risk assessment in patients with sickle cell disease. Haematologica. 2011;96:1–5. doi: 10.3324/haematol.2010.035097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;7:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 7.Chaudry RA, Cikes M, Karu T, et al. Paediatric sickle cell disease: pulmonary hypertension but normal vascular resistance. Arch Dis Child. 2011;96:131–6. doi: 10.1136/adc.2010.184028. [DOI] [PubMed] [Google Scholar]
- 8.Dham N, Ensing G, Minniti C, et al. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104:713–20. doi: 10.1016/j.amjcard.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010 May 14; doi: 10.1182/blood-2010-02-268193. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Hebbel RP. Reconstructing sickle cell disease: A data-based analysis of the “hyperhemolysis paradigm” for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol. 2001;86:123–54. doi: 10.1002/ajh.21952. [DOI] [PubMed] [Google Scholar]
- 11.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Longitudinal follow up of elevated pulmonary artery pressures in children with sickle cell disease. Br J Haematol. 2009;144:736–41. doi: 10.1111/j.1365-2141.2008.07501.x. [DOI] [PubMed] [Google Scholar]
- 12.Olnes M, Chi A, Haney C, et al. Improvement in hemolysis and pulmonary arterial systolic pressure in adult patients with sickle cell disease during treatment with hydroxyurea. Am J Hematol. 2009;84:530–2. doi: 10.1002/ajh.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordeuk VR, Campbell A, Rana S, et al. Relationship of erythropoietin, fetal hemoglobin, and hydroxyurea treatment to tricuspid regurgitation velocity in children with sickle cell disease. Blood. 2009;19:4639–44. doi: 10.1182/blood-2009-04-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rees DC. The rationale for using hydroxycarbamide in the treatment of sickle cell disease. Haematologica. 2011;96:488–91. doi: 10.3324/haematol.2011.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Beers EJ, van Eck-Smit BL, Mac Gillavry MR, et al. Large and medium-sized pulmonary artery obstruction does not play a role of primary importance in the etiology of sickle-cell disease-associated pulmonary hypertension. Chest. 2008;133:646–52. doi: 10.1378/chest.07-1694. [DOI] [PubMed] [Google Scholar]
- 16.Connor P, Veys P, Amrolia P, et al. Pulmonary hypertension in children with Evans syndrome. Pediatric Hematology Oncology. 2008;25:93–8. doi: 10.1080/08880010801888253. [DOI] [PubMed] [Google Scholar]
- 17.Steen RG, Helton KJ, Horwitz EM, et al. Improved cerebrovascular patency following therapy in patients with sickle cell disease: initial results in 4 patients who received HLA-identical hematopoietic stem cell allografts. Annals of Neurology. 2001;49:222–9. doi: 10.1002/1531-8249(20010201)49:2<222::aid-ana42>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 18.Bernaudin F, Socie G, Kuentz M, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–56. doi: 10.1182/blood-2007-03-079665. [DOI] [PubMed] [Google Scholar]
- 19.Walters MC, Hardy K, Edwards S, et al. Pulmonary, gonadal, and central nervous system status after bone marrow transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2010;16:263–72. doi: 10.1016/j.bbmt.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanaro C, Franco-Penteado CF, Albuqueque DM, et al. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leuk Biol. 2009;85:235–42. doi: 10.1189/jlb.0708445. [DOI] [PubMed] [Google Scholar]
- 21.Sata M. Role of circulating vascular progenitors in angiogenesis, vascular healing, and pulmonary hypertension: lessons from animal models. Arteriosclerosis Thrombosis and Vascular Biology. 2006;26:1008–14. doi: 10.1161/01.ATV.0000206123.94140.f3. [DOI] [PubMed] [Google Scholar]
- 22.Manci EA, Culberson DE, Yang YM, et al. Investigators of the Cooperative Study of Sickle Cell Disease. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123:359–65. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]