Abstract
By virtue of superior preservation of proteins and nucleic acids the zinc salt-based fixatives (ZBF) has been proposed as an alternative to precipitants and cross-linking fixatives in histopathology. It was recently reported that ZBF is compatible with analysis of cell surface immunophenotype and detection of intracellular epitopes by flow cytometry. The aim of this study was to explore whether ZBF is also compatible with the detection of DNA damage response assessed by phospho-specific antibodies (Abs) detecting phosphorylation of the key proteins of that pathway. DNA damage in human pulmonary adenocarcinoma A549 cells was induced by treatment with the DNA topoisomerase I inhibitor camptothecin and phosphorylation of histone H2AX on Ser139 (γH2AX) and of ATM on Ser1981 was detected with phospho-specific Abs; cellular fluorescence was measured by laser scanning cytometry (LSC). The sensitivity and accuracy of detection of H2AX and ATM phosphorylation concurrent with the detection of DNA replication by EdU incorporation and “click chemistry” was found in ZBF fixed cells to be comparable to that of cell fixed in formaldehyde. The accuracy of DNA content measurement as evident from the resolution of DNA content frequency histograms of cells stained with DAPI was somewhat better in ZBF- than in formaldehyde-fixed cells. The pattern of chromatin condensation revealed by the intensity of maximal pixel of DAPI that allows one to identify mitotic and immediately post-mitotic cells by LSC was preserved after ZBF fixation. ZBF fixation was also compatible with the detection of γH2AX foci considered to be the hallmarks of induction of DNA double-strand breaks. Analysis of cells by flow cytometry revealed that ZBF fixation of lymphoblastoid TK6 cells led to about 60 and 33% higher intensity of the side and forward light scatter, respectively, compared to formaldehyde fixed cells.
Keywords: gammaH2AX, ATM activation, click chemistry, EdU incorporation, cell cycle, DNA replication, S phase, confocal microscopy
The objective of fixation is to prevent autolysis of cellular constituents, either bacterial or fungal induced decay damage and to preserve tissue and cellular structural organization as close as possible to the in vivo state (reviews, 1–3). An optimal fixative is expected to ensure high quality histological appearance and long-term preservation of DNA, RNA, and proteins in their relatively native state. Both cell surface and intracellular proteins have to be detectable by immunocytochemical means and the samples should remain amenable to new diagnostic assays that use molecular biology tools in studies of the cell's genome and proteome (3,4).
Among the most common fixatives are the precipitants, ethanol, methanol, or acetone. Precipitants denature proteins and alter cell morphology but leave the reactive centers of many enzymes relatively unchanged. After fixative removal and hydration, the original properties of proteins, including enzymatic activity and immunoreactivity with specific antibodies (Abs), are often regained. However, many low molecular weight cellular constituents as well as glycosaminoglycans remain soluble and may leak out of the cells upon hydration. Low molecular weight DNA, the product of DNA fragmentation during apoptosis may also be extracted from the ethanol-fixed cells (5).
The second group of fixatives are the cross-linking agents formaldehyde and glutaraldehyde (1,6). They interact with the tissues by forming methylene bridges between aminoacids within individual proteins, between neighboring proteins and between aminoacids and nucleic acids. The cross-linking mechanism, although it preserves good morphology, can alter the tertiary and quaternary structure of proteins (6,7). Depending on the extent of the alteration protein structure and its accessibility, the immunocytochemical recognition of epitopes by Ab may be impeded. Cross-linking also hinders extraction of nucleic acids and proteins for analysis by PCR and Western blotting and the recovered macromolecules are chemically modified by the covalent interaction with the fixative. Furthermore, formaldehyde and glutaraldehyde liquids and fumes are highly irritating, potentially carcinogenic, and their handling requires special protection.
Zinc salt-based fixation (ZBF) has been recently proposed as an alternative to precipitating and cross-linking fixatives (4,8–11). Previous studies have shown that the preservation of nucleic acids and proteins after fixation in ZBF is superior to that obtained with buffered formalin fixation (4,9,11). In addition, cell morphology is comparable to that of formaldehyde-fixed cells and enzymatic activity of certain enzymes is preserved (12).
Jensen et al., have recently introduced ZBF fixation to flow cytometry (11). They reported that after ZBF fixation the surface immunophenotype of mouse epithelial keratinocytes expressing Sca-1, CD34, and α6 integrin was similar to that of cells fixed in formaldehyde or of unfixed live cells. They also observed that ZBF fixation is compatible with the detection of DNA replication by “click chemistry” using 5-ethynyl-2′deoxyuridine (EdU) as a DNA precursor (13) and with the immunocytochemical detection of intracellular epitopes (11). These authors were also able to extract DNA and RNA from ZBF-fixed cells and subject them to PCR and RT-PCR, respectively; their data show that both DNA and RNA were better preserved in the ZBF- compared to formaldehyde-fixed cells.
The immunocytochemical detection of protein phosphorylation with phospho-specific Abs has become a key approach to assessing the activation of many signaling pathways in individual cells by cytometry (14–16). This study, therefore, was designed to explore whether detection of epitopes by phospho-specific Ab is compatible with ZBF fixation. We have tested the detection of the two phospho-proteins, histone H2AX phosphorylated on Ser139 that is defined as γH2AX (17) and Ataxia Telangiectasia Mutated protein kinase (ATM) phosphorylated on Ser1981 (18). These phosphorylation events of H2AX and ATM are critical biomarkers of the DNA damage response and have been intensely studied by flow- and laser scanning- cytometry (reviews, 16,19,20). In this study, the DNA damage in human pulmonary adenocarcinoma A549 and human lymphoblastoid TK6 cells was induced by exposure to the DNA topoisomerase I inhibitor camptothecin (Cpt), which as was shown before (21,22) induces the damage selectively in S-phase cells. We have also explored whether phosphorylation of H2AX can be detected concurrently with the analysis of DNA replication using the “click chemistry” approach (13). The “click” method relies on the use of EdU as a DNA precursor, which once incorporated into DNA is detected with fluorochrome-tagged azides by means of a copper (I) catalyzed [312] cycloaddition reaction termed “click chemistry” (13,23). Because of the small size of the fluorescent azides they easily penetrate into the specimen. Thus, the accessibility of the EdU to the azides is greater than of BrdU to an Ab and unlike the BrdU-based methodology, no DNA denaturation is required sparing the specimen harsh treatment with strong acid or heat.
Materials and Methods
Cells, Cell Treatment
Human lung carcinoma A549 cells were purchased from American Type Culture Collection (ATCC #CCL-185, Manassas, VA). The cells were cultured in Ham's F12-K medium with 2 mM l-glutamine adjusted to contain 1.5 g/L sodium bicarbonate (ATCC) and supplemented with 10% fetal bovine serum (ATCC). Dual-chambered slides (Nunc Lab-Tek®, Thermo Fisher Scientific, Rochester, NY) were seeded with 2 ml of 105 cells/ml cell suspension per chamber 24 h before treatments with camptothecin (Cpt; Sigma-Aldrich, St. Louis, MO) and/or EdU. All incubations were at 37°C in a humidified atmosphere of 5% CO2 in air and the cells were grown to ~ 50% confluency. To induce DNA damage response the cells were treated with 0.2 μM of Cpt for 60 min. Some cultures were also treated with 5 μM EdU (Invitrogen/Molecular Probes, Eugene, OR) for 60 min. For the standard formalde-hyde fixation procedure, the cells growing on slides were immersed in 1% methanol-free formaldehyde (FM; “Ultra-pure”; Polysciences, Warrington, PA) in PBS in Coplin jars for 15 min on ice. The slides were then rinsed with PBS and transferred to 70% ethanol where they were post-fixed (stored) at −20°C for 2–24 h. Some slides were not transferred to ethanol. The cells to be fixed with ZBF were immersed in the solution as described by Jensen et al., (11) containing 0.05% calcium acetate [(CH3COO)2 Ca], 0.5% zinc acetate [(CH3COO)2 Zn], 0.5% zinc chloride [ZnCl2], and 0.1 M Tris-HCl, pH 7.8 (all from Sigma Chemical Co, St. Louis, MO) at 4°C overnight. Slides with EdU-labeled cells were stored in 1% BSA in PBS, at 4°C for up to 24 h following fixation in FM or ZBF. Human B cell lymphoblastoid TK6 cells were grown in 25 ml FALCON flasks (Becton Dickinson, Franklin Lakes, NJ) in RPMI 1640 supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (all from GIBCO/BRL Life Technologies, Grand Island, NY). These cells were fixed in suspension either in 1% FM (15 min, on ice), then rinsed and kept in 70% ethanol, or in ZBF solution at 4°C overnight. Other details are presented in Figure legends and in our recent publications (16,22).
Immunocytochemical Detection of Phosphorylated Histone H2AX (cH2AX), Activated ATM and Cell Labeling with EdU
After fixation the cells were washed twice in PBS and treated on slides with 0.1% Triton X-100 (Sigma) in PBS for 15 min, and then in a 1% (w/v) solution of bovine serum albumin (BSA; Sigma) in PBS for 30 min to suppress nonspecific antibody binding. The cells were then incubated in a 100 μl volume of PBS containing 1% (w/v) BSA and 1:300 dilution of phospho-specific (Ser139) γH2AX mAb (Biolegend, San Diego, CA) or 1:200 dilution of phospho-specific (Ser1981) ATM mAb (Millipore, Temecula, CA), for 1.5 h at room temperature or overnight at 4°C. The secondary fluoro-chrome-tagged Abs consisted of either AlexaFluor® 488 tagged Ab (Invitrogen/Molecular Probes, at 1:100 dilution) or AlexaFluor 647 tagged Ab (Invitrogen/Molecular Probes, at 1:100 dilution). Prior to measurement by laser scanning cytometry (LSC), the cells were counterstained for DNA content with 2.8 lg/ml 4,6-diamidino-2-phenylindole (DAPI; Sigma) in PBS for 15 min. Each experiment was performed with an IgG control in which cells were labeled only with the secondary antibody, AlexaFluor 488 goat anti-mouse IgG (H+L) or Alexa-Fluor 647 goat anti-rabbit IgG (H+L) without primary antibody incubation to estimate the extent of nonspecific binding of the secondary antibody to the cells. Other details of cell incubation with the primary and secondary Ab were presented before (16,18–20). To detect DNA replication the cells were incubated with 5 lM EdU for 1 h, then fixed as mentioned above. The incorporated EdU was subsequently detected by the “click chemistry” using the click-ITTM reagent kit provided by Invitrogen-Molecular Probes (Carlsbad, CA) with AlexaFluor 488 tagged azide following the protocol provided with the kit.
Measurement of Cell Fluorescence by LSC and Flow Cytometry: Detection of γH2AX Foci by Confocal Microscopy
Cellular green or far red IF representing the binding of the respective phospho-specific Abs and the blue emission of DAPI stained DNA was measured using an LSC (iCys; CompuCyte, Westwood, MA) using standard filter settings; fluorescence was excited with 488-nm argon, helium-neon (633 nm) and violet (405 nm) lasers. The intensities of maximal pixel and integrated fluorescence were measured and recorded for each cell. At least 3,000 cells were measured per sample. The forward and side light scatter of TK6 cells as well the intensity of their DAPI fluorescence was measured using the MoFlo XDP high speed flow cytometer/sorter (Beckman-Coulter, Fort Collins, CO). For confocal microscopy the untreated and Cpt treated (0.2 μM, 60 min) cells were stained with mouse anti-γH2AX (Upstate, Lake Placid; diluted 1:350); goat anti-mouse conjugated with Alexa 488 secondary antibody (InVitrogen/Molecular Probes, Eugene, OR, diluted 1:400) and counterstained with Hoechst 33342 (Sigma, Poznan, Poland). Cells were imaged using a Leica SMD confocal microscope, using standard conditions. Other details of the confocal cell examination were presented elsewhere (24,25).
RESULTS
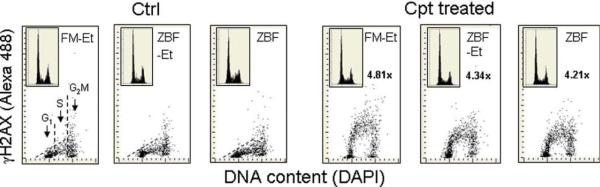
Figure 1 illustrates the detection of γH2AX in A549 cells after ZBF fixation in comparison with FM fixation. The expression of γH2AX was induced by exposure of cells to 0.2 γM Cpt for 60 min. We have shown (21,22) that such treatment triggers the DNA damage response involving phosphorylation of H2AX, p53, ATP, and Chk2 preferentially in S-phase cells. We compared ZBF fixation with our standard FM fixation which involves exposure of cells to 1% methanol-free FM dissolved in PBS for 15 min at 0–4°C, followed by rinsing in PBS and postfixation (storage) in 70% ethanol at least overnight (Fig. 1, FMEt). The pattern of H2AX phosphorylation in the untreated cells (Ctrl) reporting constitutive DNA damage response induced by endogenous oxidants (26,27), was similar in cells fixed in FM as compared to ZBF fixation, regardless of whether the ZBF fixed cells were subsequently post-fixed in ethanol or not. In addition, the pronounced expression of γH2AX in S phase of Cpt treated cells was similar regardless of fixation.
Figure 1.
Detection of γH2AX expression in A549 cells untreated or treated with DNA topo1 inhibitor camptothecin (Cpt) fixed in formaldehyde as compared with zinc-salt based fixation (ZBF). Exponentially growing A549 cells were untreated (Ctrl) or treated with 0.2 μM Cpt for 60 min, then fixed in formaldehyde followed by post- fixation in 70% ethanol (FM-Et), or fixed in ZBF followed by 70% ethanol (ZBF-Et), or fixed in ZBF with no ethanol post-fixation (ZBF). Expression of γH2AX was detected immunocytochemically using the AlexaFluor1 488 secondary Ab, DNA was counterstained with DAPI, cellular blue and green fluorescence was measured by LSC with no compensation for emission colors. The dashed skewed lines show the upper threshold of expression of γH2AX for 97% of the G1 and S-phase cells in untreated (Ctrl) cultures. The numbers in the panels report the n-fold increase in mean γH2AX immunofluorescence of the S-phase Cpt-treated cells over the mean γH2AX immunofluorescence of the S-phase of the identically fixed untreated cells. DNA content frequency histograms of the analyzed cells are shown in the respective panels. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The absolute level of the mean intensity of γH2AX IF of S-phase cells was lower after ZBF fixation compared to FM fixed cells. However, the difference between the ZBF and FM fixed cells when expressed as the n-fold increase in mean intensity of immunofluorescence (IF) of S-phase cells after Cpt treatment over the level of constitutive S-phase expression of γH2AX IF of untreated cells was less pronounced (4.34 or 4.21 vs. 4.81). This is due to the fact that the intensity of γH2AX IF of the untreated cells reporting constitutive H2AX phosphorylation was also lower (denominator) and therefore the difference when expressed in terms of the n-fold increase between the FM and ZBF fixed cells was less than the difference in the absolute level of the IF. The accuracy of DNA content measurements as reflected by the DNA content histograms, all showing low coefficients of variation of the mean DNA content of G1 cells, was comparable in ZBF and FM fixed cells (Fig. 1, insets).
The Cpt-induced ATM activation revealed by phosphorylation of Ser1981was also detectable after ZBF fixation (Fig. 2). The pattern of expression of ATM-S1981P with respect of the cell cycle phase was similar in Cpt-treated cells whether fixed with ZBF or FM; in both instances the cells responding to treatment with Cpt were predominantly in S-phase. The n-fold increase in mean intensity of the ATM-S1981P IF of Cpt-treated S-phase cells was somewhat lower following fixation with ZBF (3.17×) than with FM (4.21×).
Figure 2.

Detection of ATM phosphorylation on Ser1981 in A549 cells untreated or treated with Cpt and fixed in formaldehyde as compared with zinc-salt based fixation (ZBF). Exponentially growing A549 cells were untreated (Ctrl) or treated with 0.2 μM Cpt for 60 min, then fixed in formaldehyde (FM) or in ZBF. Expression of ATM-S1981P was detected immunocytochemically using the AlexaFluor® 647 secondary Ab, DNA was counterstained with DAPI, cellular blue and red fluorescence was measured by LSC with no compensation for emission colors. The figures in the respective panels report the n-fold increase in mean ATM-Ser1981P IF of the S-phase Cpt-treated cells over the mean ATM-Ser1981P IF of the S-phase of the identically fixed untreated cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
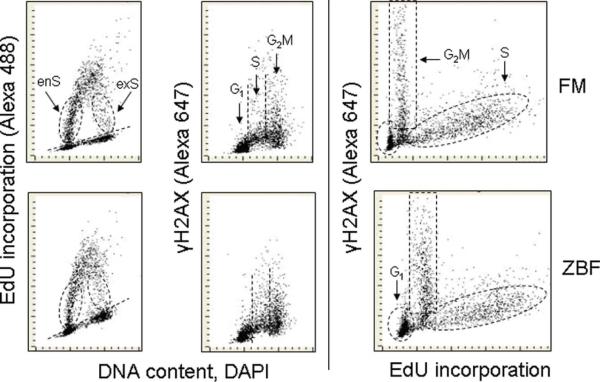
In the next experiment, we explored the effect of ZBF fixation on immunocytochemical detection of γH2AX concurrently with identification of DNA replicating cells through incorporation of 5-ethynyl-2′deoxyuridine (EdU) that was detected by the “click chemistry” using AlexaFluor tagged azide (13,23). As is shown in Figure 3 the typical bivariate distribution pattern of DNA content vs EdU incorporation is essentially identical in the ZBF and FM fixed cells. The variability in intensity of EdU incorporation makes it possible to identify the cells entering (enS) and exiting S (exS) phase during the 60 min-exposure to EdU as shown in this Figure. It is apparent that more cells were entering S phase (enS) than exiting S (exS) during this time interval. Since in asynchronous cultures the probability of detection of the cells traversing S phase sections of the same duration is inversely related to the rate of traverse through these sections (28) this would indicate that the rate of progression through the initial 60 min of S phase (initiation of DNA replication) was slower than through the final 60 min interval of S phase. Consistent with our prior observation (26,27) the data show that G2M-phase cells have distinctly higher level of γH2AX expression than G1 or most S-phase cells.
Figure 3.
Detection of EdU incorporation by the “click chemistry” approach (24) combined with immunocytochemical detection of the constitutively expressed γH2AX in cells fixed with formaldehyde and ZBF. Exponentially growing A549 cells were exposed for 60 min to EdU then fixed either in FM (top panels) or in ZBF (bottom panels).The dashed skewed lines (left panels) show the threshold separating EdU incorporating from non-incorporating cells. The cells entering (enS) and exiting S (exS) phase during the 60 min-exposure to EdU show variable intensity of EdU incorporation. The scales of the γH2AX and EdU coordinates in right panels are expanded compared to the left panel and mid panels to better visualize the relationship between expression of γH2AX and EdU incorporation at comparable relative scale. Note the similarity of the patterns of EdU incorporation versus DNA content or γH2AX IF versus EdU incorporation regardless of fixation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We have also tested whether ZBF fixation is compatible with the detection of γH2AX foci, considered to be the hallmarks of the induction of DNA double-strand breaks (17). As it is evident in Figure 4 whether the cells were fixed in FM or ZBF the foci could be easily identified in the A549 Cpt-treated cells by confocal microscopy.
Figure 4.
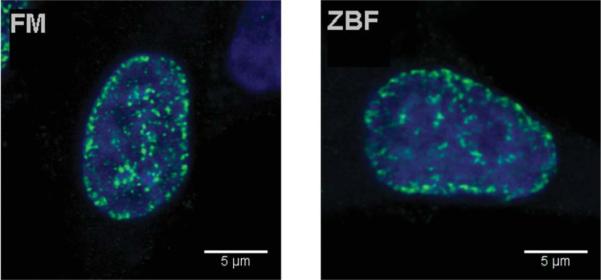
Single confocal planes demonstrating γH2AX foci in A549 cells treated with Cpt and fixed in FM or ZBF. There is no substantial difference between the appearance of foci after both methods of fixation.
The imaging capabilities of LSC (29) have been used to assess how ZBF fixation affects morphometric attributes of the cell. Towards this end, we have compared the maximal pixel of DAPI fluorescence versus nuclear area of cells fixed in FM and in ZBF. As is shown in Figure 5 these distributions are rather similar, but relatively minor increases in maximal pixel and a decrease in nuclear area were seen after ZBF fixation. Using the imaging and the “CompuSort” capabilities of the LSC (29–31) we were able to identify mitotic (M) and post-mitotic (pM) cells as the cells with the highest intensity of maximal pixel (31) reflecting their high degree of chromatin condensation, both after fixation in FM as well as in ZBF (imaged through the M and pM oval gates as shown in Fig. 5).
Figure 5.
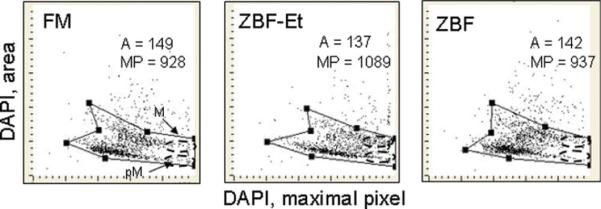
The bivariate distributions of nuclear (DAPI) area versus DAPI fluorescence intensity of maximal pixel of A549 cells fixed with formaldehyde as compared with ZBF. The cells were fixed either in formaldehyde (FM), in ZBF followed by 70% ethanol (ZBF-Et), or only in ZBF then stained with DAPI and their fluorescence was measured by LSC. The figures show the mean area size (A) and mean intensity of maximal pixel (MP; both in arbitrary units) for the population of cells gated as shown. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We have also assessed the detection of γH2AX in cells fixed in ZBF by flow cytometry as well as compared the light scatter properties of cells fixed in ZBF and FM (Fig. 6). In this experi ment, the human lymphoblastoid TK6 cells, untreated or treated with 0.2 μM Cpt for 60 min were fixed in suspension, their γH2AX was detected with the secondary Ab tagged with Alexa-Fluor 488, DNA counterstained with DAPI and the cells were analyzed by the MoFlo XDP high speed flow cytometer/sorter. It is quite evident that the induction of γH2AX by Cpt is detectable in ZBF-fixed cells. It is also apparent that intensity of the right angle (side) light scatter as well as the forward light scatter was higher in ZBF-fixed cells compared to FM-fixed cells. Specifically, for the cell population marked with the oval boundaries in Figure 6 the mean value of intensity of side scatter was 61%- and of forward scatter was 33%- higher after ZBF - than after FM- fixation. The DNA content frequency histograms show better accuracy of DNA content measurement in the ZBF fixed cells whose CV for the G1 cell population was 4.5 compared with 6.9 for the FM-fixed cells.
Figure 6.
Light scatter and DNA content frequency histograms of TK6 cells fixed in formaldehyde (FM) or ZBF (two left panels) and expression of γH2AX in untreated (Ctrl) or Cpt-treated cells, fixed in ZBF (right panels), measured by flow cytometry. Half-peak coefficient of variation (CV) of the mean of DNA content of the G1 cells (CV) is shown in the respective panels. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
This data demonstrate that ZBF fixation is fully compatible with the immunocytochemical detection of epitopes of phospo-proteins histone H2AX phosphorylated on Ser139 and ATM phosphorylated on Ser1981, the key biomarkers of DNA damage response. The intensity of the fluorescence signals of these phos phoproteins in the ZBF fixed cells, regardless of whether the cells were post-fixed (preserved) in 70% ethanol, or not, was comparable to that of the cells fixed in a standard way, in ethanol-free FM. It should be noted that the cells that were not post-fixed in ethanol were permeabilized by exposure to Triton-X-100. Confirming the findings of Jensen et al., (11) we observed that detection of DNA replication through incorporation of EdU followed by click chemistry (13) was also fully compatible with ZBF fixation.
The presence of the immunocytochemically revealed γH2AX foci was detected in the Cpt-treated cells fixed in ZBF, which was similar to that of cells fixed in FM (Fig. 4). Interestingly, the pattern of distribution of γH2AX foci resembled very much the pattern of DNA replication factories being detected by the incorporation of fluorochrome-tagged DNA precursors, shown to vary depending on the time-window within the S-phase (32–34). The pattern seen in Figure 4, with the presence of γH2AX foci preferentially distributed at the nuclear periphery and around nucleoli, is almost identical to that of DNA replication sites at the mid-S phase, as presented by Gillespie and Blow (34). Since Cpt is known to induce DNA damage selectively in DNA replication cells (21,22,35) it is not surprising to observe that the pattern of expression of γH2AX is almost identical to that of DNA replication factories. Studies are in progress to directly correlate, within the same cells, the localization DNA replication factories detected by EdU incorporation with the induction of γH2AX and phosphorylation of ATM (Dobrucki et al., in preparation).
This is the first report on the analysis of ZBF fixed cells by LSC. The morphometric features of the cells related to the degree of chromatin condensation, which make it possible to identify mitotic and post-mitotic cells based on intensity of the maximal pixel of DNA-associated (DAPI) fluorescence (29,31) were similar following both ZBF and FM fixation (Fig. 5). Thus, we were able to identify these cells based on their morphology after “CompuSort” and imaging.
As mentioned, one advantage of ZBF, particularly when compared with FM or glutaraldehyde, is low toxicity. Unlike the later, fixation with ZBF does not necessitate the use of fume hoods and other means of special protection. In addition, knowledge of the chemical composition and low cost is an advantage of ZBF when comparing it to the nontoxic fixatives provided by vendors that do not reveal their contents (36,37). Without information on the chemical composition of the fixative, it may be impossible to determine whether, for example, a failure of an experiment may be caused by the presence of a particular constituent of the fixative.
Little is known about the mechanism of cell fixation in ZBF. It has been suggested that the presence of acetate ions in the ZBF solution provides mechanism of chaotropic interactions (11). According to Wikipedia, chaotropic agents interfere with stabilizing intramolecular interactions mediated by non-covalent forces such as hydrogen bonds, van der Waals forces, and hydrophobic interactions. However, Zn ions are known to stabilize the tertiary structure of proteins and thus may counteract the effects of acetate. The authors suggest that a combination of acetate and Zn ions enforces structural changes including mild denaturation of proteins, which may congeal proteins in the plasma membrane (11). Regardless of the mechanism, ZBF offers superior preservation of proteins, DNA and RNA (4,11). As the data of Jensen et al. (11) and our present findings show, ZBF is fully compatible with the analysis of individual cells by flow- and imaging- cytometry. One would expect that ZBF may be of particular value in experiments designed to sort desired cell subpopulations identified by immunophenotype for their further analysis by molecular biology methods such as PCR, RT-PCR, Western blotting, and proteome cell analysis.
Acknowledgments
Grant sponsor: NCI; Grant number: R01 28 704; Grant sponsor: Polish Ministry of Science and Higher Education; Grant numbers: POIG.01.01.02-00-109/09 and POIG 02.01.00-12-064/08
Literature Cited
- 1.Pearse AGE. Theoretical and Applied. 3rd ed. Vol.1. Little, Brown & Company; Boston, MA: 1968. Histochemistry. [Google Scholar]
- 2.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. Churchill Livingstone; London, UK: 2002. [Google Scholar]
- 3.Paavilainen L, Edvinsson Å , Asplund A, Hober S, Kampf C, Pontén F, Wester K. The impact of tissue fixatives on morphology and antibody-based protein profiling in tissues and cells. J Histochem Cytochem. 2010;58:237–246. doi: 10.1369/jhc.2009.954321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lykides D, Van Noorden S, Armstrong A, Spencer-Dene B, Li j, Zhuang Z, Stamp GWH. Novel zinc-based fixative for high quality DNA, RNA and protein analysis. Nucleic Acids Res. 2007;35:e85. doi: 10.1093/nar/gkm433. Epub 2007 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong JP, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994;218:314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- 6.Puchtler H, Mcloan SN. On the chemistry of formaldehyde fixation and its effects on immunohistochemical reactions. Histochemistry. 1985;82:201–204. doi: 10.1007/BF00501395. [DOI] [PubMed] [Google Scholar]
- 7.Helander KG. Kinetic studies of formaldehyde binding in tissue. Biotech Histochem. 1994;69:177–179. doi: 10.3109/10520299409106282. [DOI] [PubMed] [Google Scholar]
- 8.Beckstead JH. A simple technique for preservation of fixation sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem. 1994;42:1127–1134. doi: 10.1177/42.8.8027531. [DOI] [PubMed] [Google Scholar]
- 9.Wester K, Asplund A, Baäckvall H, Micke P, Derveniece A, Hartmane I, Malmström PU, Ponten F. Zinc-based fixative improves preservation of genomic DNA and peroteins in histoprocessing of human tissues. Lab Invest. 2003;83:889–899. doi: 10.1097/01.lab.0000074892.53211.a5. [DOI] [PubMed] [Google Scholar]
- 10.Hicks DJ, Johnson L, Mitchell SM, Gough J, Cooley WA, La Ragone RM, Spencer YI, Wangoo A. Evaluation of zinc salt based fixatives for preserving antigenic determinants for immunocytochemical demonstration of murine immune system cell markers. Biotech Histochem. 2006;81:23–30. doi: 10.1080/10520290600725375. [DOI] [PubMed] [Google Scholar]
- 11.Jensen UB, Owens DM, Pedersen S, Christensen R. Zinc fixation preserves flow cytometry scatter and fluorescence parameters and allows simultaneous analysis of DNA content and synthesis, and intracellular and surface phenotypes. Cytometry Part A. 2010;77A:798–804. doi: 10.1002/cyto.a.20914. [DOI] [PubMed] [Google Scholar]
- 12.Hadler-Olsen E, Kanapathippillai P, Berg E, Svineng G, Winberg JO, Uhlin-Hansen L. Gelatin in situ zymography on fixed, paraffin-embedded tissue: Zinc and ethanol fixation preserves enzyme activity. J Histochem Cytochem. 2010;58:29–39. doi: 10.1369/jhc.2009.954354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juan G, Gruenwald S, Darzynkiewicz Z. Phosphorylation of retinoblastoma susceptibility gene protein assayed in individual lymphocytes during their mitogenic stimulation. Exp Cell Res. 1998;239:104–110. doi: 10.1006/excr.1997.3885. [DOI] [PubMed] [Google Scholar]
- 15.Chow S, Minden MD, Hedley DW. Constitutive phosphorylation of the S6 ribosomal protein via mTOR and ERK signaling in the peripheral blasts of acute leukemia patients. Exp Hematol. 2006;34:1183–1191. doi: 10.1016/j.exphem.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Traganos F, Dobrucki J, Wlodkowic D, Darzynkiewicz Z. Induction of DNA damage response by the supravital probes of nucleic acids. Cytometry Part A. 2009;75A:510–519. doi: 10.1002/cyto.a.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedelnikova OA, Rogakou EP, Panuytin IG, Bonner W. Quantitative detection of 125IUdr-induced DNA double-strand breaks with γ-H2AX antibody. Radiat Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka T, Huang X, Halicka HD, Zhao H, Traganos F, Albino AP, Dai W, Darzynkiewicz Z. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry Part A. 2007;71A:648–661. doi: 10.1002/cyto.a.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Halicka HD, Traganos F, Tanaka T, Kurose A, Darzynkiewicz Z. Cytometric assessment of DNA damage in relation to cell cycle phase and apoptosis. Cell Prolif. 2005;38:223–243. doi: 10.1111/j.1365-2184.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, Okafuji M, Traganos F, Luther E, Holden E, Darzynkiewicz Z. Assessment of histone H2AX phosphorylation induced by DNA topoisomerase I and II inhibitors topotecan and mitoxantrone and by DNA crosslinking agent cisplatin. Cytometry Part A. 2004;58A:99–110. doi: 10.1002/cyto.a.20018. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Traganos F, Darzynkiewicz Z. Phosphorylation of p53 on Ser15 during cell cycle caused by Topo I and Topo II inhibitors in relation to ATM and Chk2 activation. Cell Cycle. 2008;7:3048–3055. doi: 10.4161/cc.7.19.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darzynkiewicz Z, Traganos F, Zhao H, Halicka HD, Li J. Cytometry of DNA replication and RNA synthesis. Historical perspective and recent advances based on “click chemistry.”. Cytometry Part A. 2011;79A:328–337. doi: 10.1002/cyto.a.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Oczos J, Janowski P, Trembecka D, Dobrucki J, Darzynkiewicz Z, Wlodkowic D. Rationale for the real-time and dynamic cell death assays using propidum iodide. Cytometry Part A. 2010;77A:399–405. doi: 10.1002/cyto.a.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobrucki J, Darzynkiewicz Z. Chromatin condensation and sensitivity of DNA in situ to denaturation during cell cycle and apoptosis. A confocal microscopy study. Micron. 2001;32:645–652. doi: 10.1016/s0968-4328(00)00069-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Tanaka T, Halicka HD, Traganos F, Zarebski M, Dobrucki J, Darzynkiewicz Z. Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports the aging-related processes. Cytometry Part A. 2007;71A:905–914. doi: 10.1002/cyto.a.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka T, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle. 2006;5:1940–1945. doi: 10.4161/cc.5.17.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darzynkiewicz Z. Mammalian cell-cycle analysis. In: Fantes P, Brooks R, editors. The Cell Cycle. A Practical Approach. Oxford University Press; Oxford, New York: 1993. pp. 45–68. [Google Scholar]
- 29.Pozarowski P, Holden E, Darzynkiewicz Z. Laser scanning cytometry: Principles and applications. Methods Mol Biol. 2006;319:65–192. doi: 10.1007/978-1-59259-993-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H, Halicka HD, Jorgensen E, Traganos F, Darzynkiewicz Z. New biomarkers probing the depth of cell senescence assessed by laser scanning cytometry. Cytometry Part A. 2010;77A:999–1007. doi: 10.1002/cyto.a.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luther E, Kamentsky LA. Resolution of mitotic cells using laser scanning cytometry. Cytometry. 1996;23:272–278. doi: 10.1002/(SICI)1097-0320(19960401)23:4<272::AID-CYTO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Melamed MR, Darzynkiewicz Z. Detection of apoptosis and DNA replication by differential labeling of DNA strand breaks with fluorochromes of different color. Exp Cell Res. 1996;222:28–37. doi: 10.1006/excr.1996.0004. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura E, Blow JJ, Tanaka TU. Live-cell imaging reveals replication of individual replicons in eukaryotic replication factories. Cell. 2006;125:1297–1308. doi: 10.1016/j.cell.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillespie PJ, Blow JJ. Clusters, factories and domains. The complex structure of S-phase comes into focus. Cell Cycle. 2010;9:3218–3226. doi: 10.4161/cc.9.16.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Bino G, Lassota P, Darzynkiewicz Z. The S-phase cytotoxicity of camptothecin. Exp Cell Res. 1991;193:27–35. doi: 10.1016/0014-4827(91)90534-2. [DOI] [PubMed] [Google Scholar]
- 36.Olert J, Wiedorn KH, Goldmann T, Kuhl H, Mehrain Y, Scherthan H, Niketaghad F, Vollmer E, Muller AM, Muller-Navia J. HOPE fixation: A novel fixing method and paraffin-embedding technique for human soft tissues. Pathol Res Pract. 2001;197:823–826. doi: 10.1078/0344-0338-00166. [DOI] [PubMed] [Google Scholar]
- 37.Vollmer E, Galle J, Lang DS, Loeschke S, Schultz H, Goldman T. The HOPE technique opens up a multitude of new possibilities in pathology. Rom J Morphol Embryol. 2006;47:15–19. [PubMed] [Google Scholar]