Abstract
We describe the design and synthesis of nonpeptidal antagonists of the peptide hormone cholecystokinin. Several of these compounds have high specificity and nanomolar binding affinity and are active after oral administration. To our knowledge, the design of such agents has not previously been accomplished for any peptide hormone. The structural similarities between these synthetic compounds and the anxiolytic 1,4-benzodiazepines are noted, and the potential of this structural feature for future design of ligands for other peptide hormone receptors is discussed.
Full text
PDF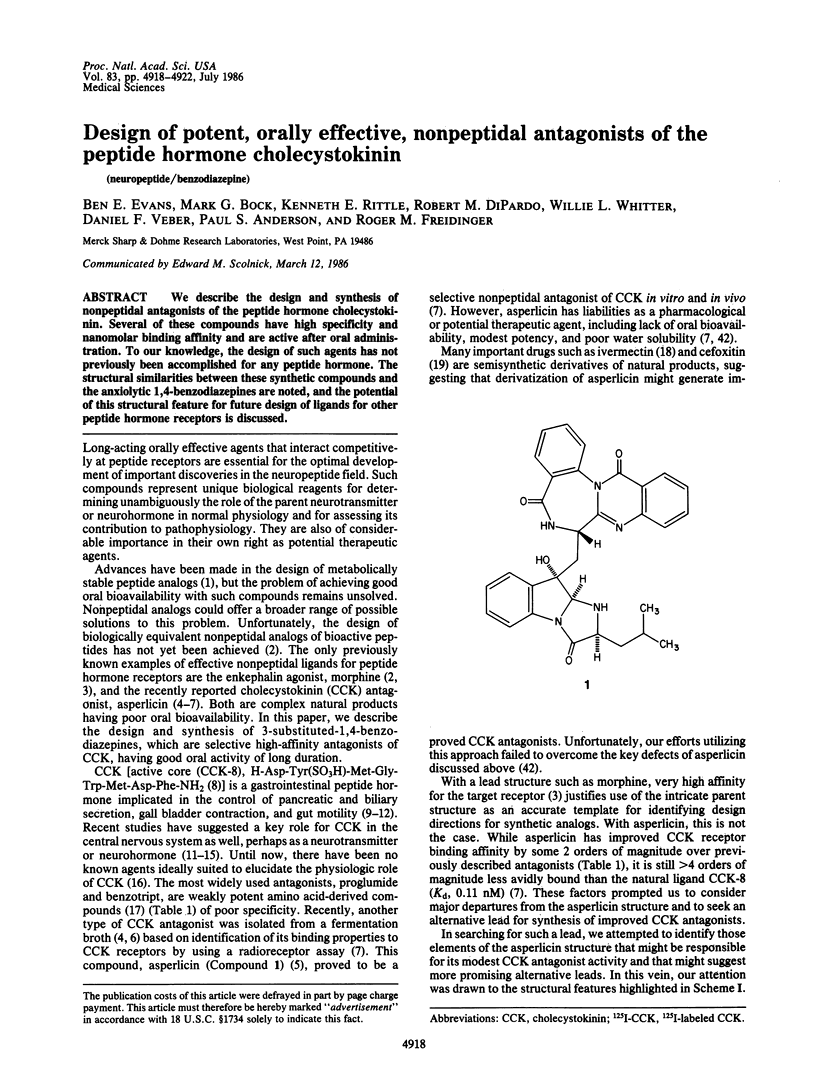


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alho H., Costa E., Ferrero P., Fujimoto M., Cosenza-Murphy D., Guidotti A. Diazepam-binding inhibitor: a neuropeptide located in selected neuronal populations of rat brain. Science. 1985 Jul 12;229(4709):179–182. doi: 10.1126/science.3892688. [DOI] [PubMed] [Google Scholar]
- Beinfeld M. C. Cholecystokinin in the central nervous system: a minireview. Neuropeptides. 1983 Oct;3(6):411–427. doi: 10.1016/0143-4179(83)90032-x. [DOI] [PubMed] [Google Scholar]
- Bell S. C., McCaully R. J., Gochman C., Childress S. J., Gluckman M. I. 3-substituted 1,4-benzodiazepin-2-ones. J Med Chem. 1968 May;11(3):457–461. doi: 10.1021/jm00309a010. [DOI] [PubMed] [Google Scholar]
- Bradwejn J., de Montigny C. Benzodiazepines antagonize cholecystokinin-induced activation of rat hippocampal neurones. Nature. 1984 Nov 22;312(5992):363–364. doi: 10.1038/312363a0. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Squires R. F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabala J. C., Mrozik H., Tolman R. L., Eskola P., Lusi A., Peterson L. H., Woods M. F., Fisher M. H., Campbell W. C., Egerton J. R. Ivermectin, a new broad-spectrum antiparasitic agent. J Med Chem. 1980 Oct;23(10):1134–1136. doi: 10.1021/jm00184a014. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Monaghan R. L., Birnbaum J., Stapley E. O., Goetz M. A., Albers-Schönberg G., Patchett A. A., Liesch J. M., Hensens O. D. A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from Aspergillus alliaceus. Science. 1985 Oct 11;230(4722):177–179. doi: 10.1126/science.2994227. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. The physiology of cholecystokinin in brain and gut. Br Med Bull. 1982 Sep;38(3):253–258. doi: 10.1093/oxfordjournals.bmb.a071769. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Jensen R. T. Cholecystokinin receptor antagonists. Am J Physiol. 1984 May;246(5 Pt 1):G471–G476. doi: 10.1152/ajpgi.1984.246.5.G471. [DOI] [PubMed] [Google Scholar]
- Goetz M. A., Lopez M., Monaghan R. L., Chang R. S., Lotti V. J., Chen T. B. Asperlicin, a novel non-peptidal cholecystokinin antagonist from Aspergillus alliaceus. Fermentation, isolation and biological properties. J Antibiot (Tokyo) 1985 Dec;38(12):1633–1637. doi: 10.7164/antibiotics.38.1633. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne W. F., Jensen R. T., Lemp G. F., Gardner J. D. Proglumide and benzotript: members of a different class of cholecystokinin receptor antagonists. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6304–6308. doi: 10.1073/pnas.78.10.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester J. B., Jr, Rudzik A. D., Kamdar B. V. 6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines which have central nervous system depressant activity. J Med Chem. 1971 Nov;14(11):1078–1081. doi: 10.1021/jm00293a015. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Snyder S. H. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstens P. J., Lamers C. B., Jansen J. B., de Jong A. J., Hessels M., Hafkenscheid J. C. Physiological plasma concentrations of cholecystokinin stimulate pancreatic enzyme secretion and gallbladder contraction in man. Life Sci. 1985 Feb 11;36(6):565–569. doi: 10.1016/0024-3205(85)90638-1. [DOI] [PubMed] [Google Scholar]
- Kubota K., Sugaya K., Matsuda I., Matsuoka Y., Terawaki Y. Reversal of antinociceptive effect of cholecystokinin by benzodiazepines and a benzodiazepine antagonist, Ro 15-1788. Jpn J Pharmacol. 1985 Jan;37(1):101–105. doi: 10.1254/jjp.37.101. [DOI] [PubMed] [Google Scholar]
- Kubota K., Sugaya K., Sunagane N., Matsuda I., Uruno T. Cholecystokinin antagonism by benzodiazepines in the contractile response of the isolated guinea-pig gallbladder. Eur J Pharmacol. 1985 Apr 2;110(2):225–231. doi: 10.1016/0014-2999(85)90215-8. [DOI] [PubMed] [Google Scholar]
- Liesch J. M., Hensens O. D., Springer J. P., Chang R. S., Lotti V. J. Asperlicin, a novel non-peptidal cholecystokinin antagonist from Aspergillus alliaceus. Structure elucidation. J Antibiot (Tokyo) 1985 Dec;38(12):1638–1641. doi: 10.7164/antibiotics.38.1638. [DOI] [PubMed] [Google Scholar]
- Morley J. E. Minireview. The ascent of cholecystokinin (CCK) - from gut to brain. Life Sci. 1982 Feb 7;30(6):479–493. doi: 10.1016/0024-3205(82)90261-2. [DOI] [PubMed] [Google Scholar]
- Möhler H., Okada T., Heitz P., Ulrich J. Biochemical identification of the site of action of benzodiazepines in human brain by 3H-diazepam binding. Life Sci. 1978 Mar;22(11):985–995. doi: 10.1016/0024-3205(78)90364-8. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Pluscec J., Sabo E. F., Sheehan J. T., Williams N. Synthesis of cholecystokinin-pancreozymin. I. The C-terminal dodecapeptide. J Am Chem Soc. 1970 Jan 14;92(1):195–199. doi: 10.1021/ja00704a033. [DOI] [PubMed] [Google Scholar]
- Rajh H. M., Mariman E. C., Tesser G. I., Nivard R. J. Tryptophan replacement in the C-terminal octapeptide of CCK-PZ. Syntheses of six peptide analogues bearing tryptophan congeners. Int J Pept Protein Res. 1980 Mar;15(3):200–210. doi: 10.1111/j.1399-3011.1980.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Sunjić V., Kuftinec J., Kajfez F. Chiral 1,4-benzodiazepine. VII. Cyclization rates of 2-(n-alpha-ammoniumacyl)-amino-5-chloro-benzophenones in the chiral 1,4-benzodiazepin-2-ones. Arzneimittelforschung. 1975 Mar;25(3):340–343. [PubMed] [Google Scholar]


