Abstract
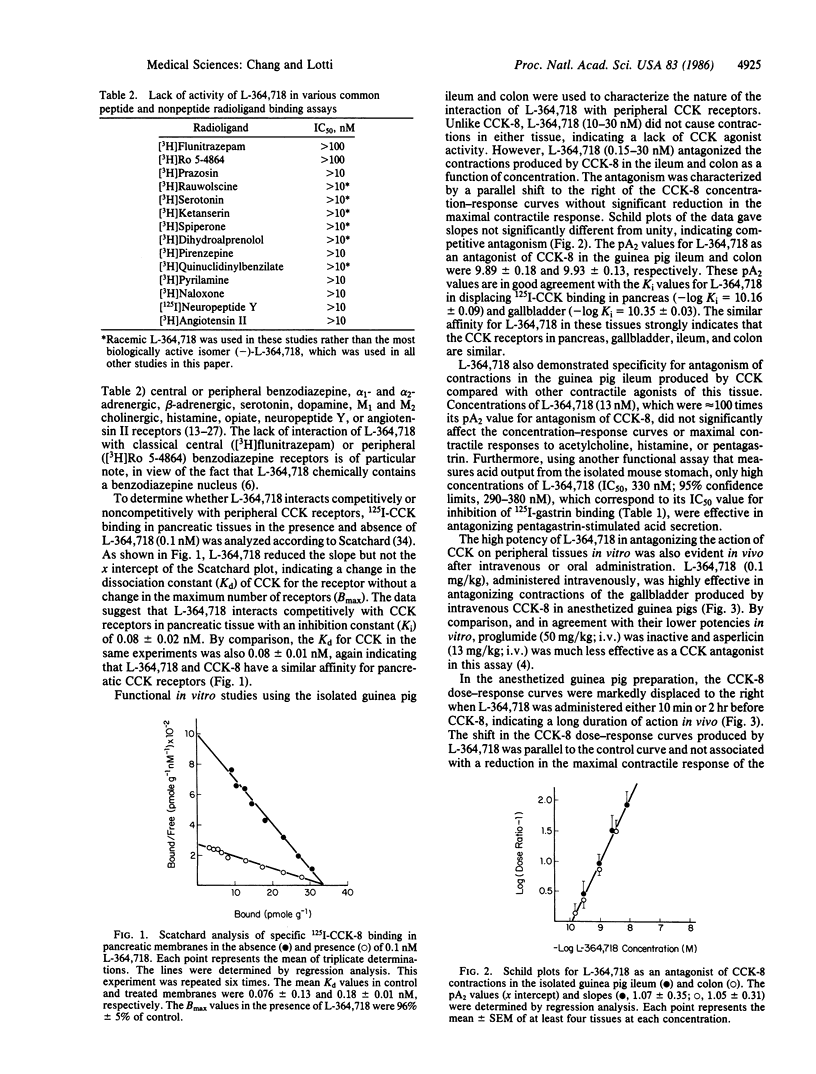
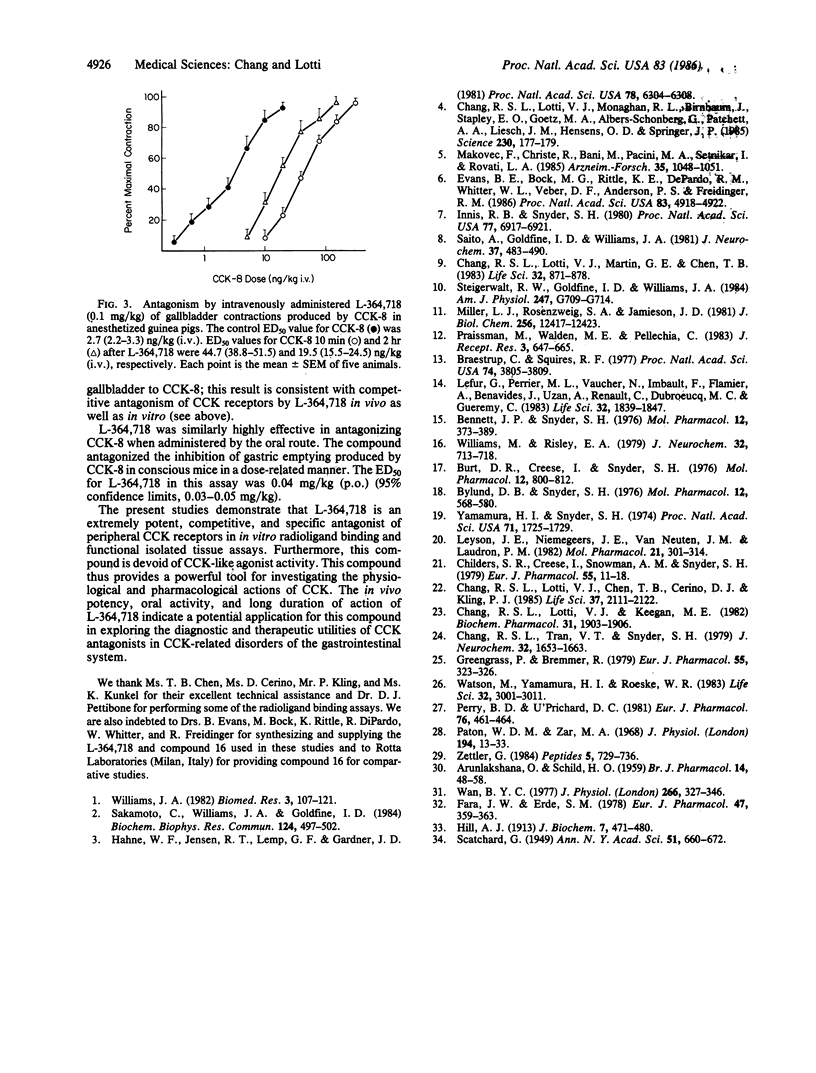
3S(-)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4- benzodiazepine-3-yl)-1H-indole-2-carboxamide (L-364,718) interacted in a competitive manner with rat pancreatic cholecystokinin (CCK) receptors as determined by Scatchard analysis of the specific binding of 125I-labeled CCK. The affinity of L-364,718 for both pancreatic (IC50, 81 pM) and gallbladder (IC50, 45 pM) CCK receptors in radioligand binding assays greatly exceeded that of other reported nonpeptide CCK antagonists and was similar to that of CCK itself. In vitro functional studies utilizing CCK-induced contractions of the isolated guinea pig ileum and colon further demonstrated that L-364,718 acts as a competitive CCK antagonist, which lacks agonist activity and has a similar high affinity in these tissues (pA2, 9.9). L-364,718 exhibited a very high selectivity for peripheral CCK receptors relative to brain CCK, gastrin, and various other peptide and nonpeptide receptors in both in vitro radioligand and isolated tissue assays. In vivo, low intravenous doses of L-364,718 (0.1 mg/kg) markedly antagonized the contractions of the guinea pig gallbladder produced by intravenous administration of CCK for at least 2 hr. Administered orally, L-364,718 (ED50, 0.04 mg/kg) was highly effective as an antagonist of CCK-induced inhibition of gastric emptying in mice. The biochemical and pharmacological properties of L-364,718--namely, very high affinity and selectivity for peripheral CCK receptors, long-lasting in vivo efficacy, and oral bioavailability--makes this compound a powerful tool for investigating the physiological and pharmacological actions of CCK, and possibly its role in gastrointestinal disorders.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Jr, Snyder S. H. Serotonin and lysergic acid diethylamide binding in rat brain membranes: relationship to postsynaptic serotonin receptors. Mol Pharmacol. 1976 May;12(3):373–389. [PubMed] [Google Scholar]
- Braestrup C., Squires R. F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt D. R., Creese I., Snyder S. H. Properties of [3H]haloperidol and [3H]dopamine binding associated with dopamine receptors in calf brain membranes. Mol Pharmacol. 1976 Sep;12(5):800–812. [PubMed] [Google Scholar]
- Bylund D. B., Snyder S. H. Beta adrenergic receptor binding in membrane preparations from mammalian brain. Mol Pharmacol. 1976 Jul;12(4):568–580. [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Chen T. B., Cerino D. J., Kling P. J. Neuropeptide Y (NPY) binding sites in rat brain labeled with 125I-Bolton-Hunter NPY: comparative potencies of various polypeptides on brain NPY binding and biological responses in the rat vas deferens. Life Sci. 1985 Dec 2;37(22):2111–2122. doi: 10.1016/0024-3205(85)90583-1. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Keegan M. E. Inactivation of angiotensin II receptors in bovine adrenal cortex by dithiothreitol: further evidence for the essential nature of disulfide bonds. Biochem Pharmacol. 1982 May 15;31(10):1903–1906. doi: 10.1016/0006-2952(82)90495-6. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Martin G. E., Chen T. B. Increase in brain 125I-cholecystokinin (CCK) receptor binding following chronic haloperidol treatment, intracisternal 6-hydroxydopamine or ventral tegmental lesions. Life Sci. 1983 Feb 21;32(8):871–878. doi: 10.1016/0024-3205(83)90224-2. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Monaghan R. L., Birnbaum J., Stapley E. O., Goetz M. A., Albers-Schönberg G., Patchett A. A., Liesch J. M., Hensens O. D. A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from Aspergillus alliaceus. Science. 1985 Oct 11;230(4722):177–179. doi: 10.1126/science.2994227. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Tran V. T., Snyder S. H. Heterogeneity of histamine H1-receptors: species variations in [3H]mepyramine binding of brain membranes. J Neurochem. 1979 Jun;32(6):1653–1663. doi: 10.1111/j.1471-4159.1979.tb02276.x. [DOI] [PubMed] [Google Scholar]
- Childers S. R., Creese I., Snowman A. M., Synder S. H. Opiate receptor binding affected differentially by opiates and opioid peptides. Eur J Pharmacol. 1979 Apr 1;55(1):11–18. doi: 10.1016/0014-2999(79)90142-0. [DOI] [PubMed] [Google Scholar]
- Evans B. E., Bock M. G., Rittle K. E., DiPardo R. M., Whitter W. L., Veber D. F., Anderson P. S., Freidinger R. M. Design of potent, orally effective, nonpeptidal antagonists of the peptide hormone cholecystokinin. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4918–4922. doi: 10.1073/pnas.83.13.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fara J. W., Erde S. M. Comparison of in vivo and in vitro responses to sulfated and non-sulfated ceruletide. Eur J Pharmacol. 1978 Feb 1;47(3):359–363. doi: 10.1016/0014-2999(78)90244-3. [DOI] [PubMed] [Google Scholar]
- Greengrass P., Bremner R. Binding characteristics of 3H-prazosin to rat brain alpha-adrenergic receptors. Eur J Pharmacol. 1979 May 1;55(3):323–326. doi: 10.1016/0014-2999(79)90202-4. [DOI] [PubMed] [Google Scholar]
- Hahne W. F., Jensen R. T., Lemp G. F., Gardner J. D. Proglumide and benzotript: members of a different class of cholecystokinin receptor antagonists. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6304–6308. doi: 10.1073/pnas.78.10.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Snyder S. H. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Fur G., Perrier M. L., Vaucher N., Imbault F., Flamier A., Benavides J., Uzan A., Renault C., Dubroeucq M. C., Guérémy C. Peripheral benzodiazepine binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide. I. In vitro studies. Life Sci. 1983 Apr 18;32(16):1839–1847. doi: 10.1016/0024-3205(83)90062-0. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Niemegeers C. J., Van Nueten J. M., Laduron P. M. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982 Mar;21(2):301–314. [PubMed] [Google Scholar]
- Makovec F., Chistè R., Bani M., Pacini M. A., Setnikar I., Rovati L. A. New glutaramic acid derivatives with potent competitive and specific cholecystokinin-antagonistic activity. Arzneimittelforschung. 1985;35(7):1048–1051. [PubMed] [Google Scholar]
- Miller L. J., Rosenzweig S. A., Jamieson J. D. Preparation and characterization of a probe for the cholecystokinin octapeptide receptor, N alpha (125I-desaminotyrosyl)CCK-8, and its interactions with pancreatic acini. J Biol Chem. 1981 Dec 10;256(23):12417–12423. [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B. D., U'Prichard D. C. [3H]rauwolscine (alpha-yohimbine): a specific antagonist radioligand for brain alpha 2-adrenergic receptors. Eur J Pharmacol. 1981 Dec 17;76(4):461–464. doi: 10.1016/0014-2999(81)90123-0. [DOI] [PubMed] [Google Scholar]
- Praissman M., Walden M. E., Pellecchia C. Identification and characterization of a specific receptor for cholecystokinin on isolated fundic glands from guinea pig gastric mucosa using a biologically active 125I-CCK-8 probe. J Recept Res. 1983;3(5):647–665. doi: 10.3109/10799898309041952. [DOI] [PubMed] [Google Scholar]
- Saito A., Goldfine I. D., Williams J. A. Characterization of receptors for cholecystokinin and related peptides in mouse cerebral cortex. J Neurochem. 1981 Aug;37(2):483–490. doi: 10.1111/j.1471-4159.1981.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto C., Williams J. A., Goldfine I. D. Brain CCK receptors are structurally distinct from pancreas CCK receptors. Biochem Biophys Res Commun. 1984 Oct 30;124(2):497–502. doi: 10.1016/0006-291x(84)91581-x. [DOI] [PubMed] [Google Scholar]
- Steigerwalt R. W., Goldfine I. D., Williams J. A. Characterization of cholecystokinin receptors on bovine gallbladder membranes. Am J Physiol. 1984 Dec;247(6 Pt 1):G709–G714. doi: 10.1152/ajpgi.1984.247.6.G709. [DOI] [PubMed] [Google Scholar]
- Wan B. Y. Metiamide and stimulated acid secretion from the isolated non-distended and distended mouse stomach. J Physiol. 1977 Apr;266(2):327–346. doi: 10.1113/jphysiol.1977.sp011770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M., Yamamura H. I., Roeske W. R. A unique regulatory profile and regional distribution of [3H]pirenzepine binding in the rat provide evidence for distinct M1 and M2 muscarinic receptor subtypes. Life Sci. 1983 Jun 27;32(26):3001–3011. doi: 10.1016/0024-3205(83)90652-5. [DOI] [PubMed] [Google Scholar]
- Williams M., Risley E. A. Characterization of the binding of [3H]muscimol, a potent gamma-aminobutyric acid agonist, to rat brain synaptosomal membranes using a filtration assay. J Neurochem. 1979 Mar;32(3):713–718. doi: 10.1111/j.1471-4159.1979.tb04553.x. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetler G. Ceruletide, ceruletide analogues and cholecystokinin octapeptide (CCK-8): effects on isolated intestinal preparations and gallbladders of guinea pigs and mice. Peptides. 1984 Jul-Aug;5(4):729–736. doi: 10.1016/0196-9781(84)90014-7. [DOI] [PubMed] [Google Scholar]


