Abstract
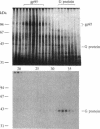
The sense of smell involves the stimulation of sensory neurons by odorants to produce depolarization and action potentials. We show that olfactory responses may be mediated by a GTP-binding protein (G protein), a homolog of the visual, hormonal, and brain signal transducing polypeptides. The olfactory G protein is identified in isolated dendritic membranes (olfactory cilia preparations) of chemosensory neurons from three vertebrate species and is shown to mediate the stimulation by odorants of the highly active adenylate cyclase in these membranes. The G protein of olfactory neurons is most similar to Gs, the hormonal stimulatory GTP-binding protein. Its alpha subunit has a molecular weight of about 42,000, and it undergoes ADP-ribosylation catalyzed by cholera toxin that leads to adenylate cyclase activation. The slight difference in molecular weights of the frog olfactory and the liver Gs alpha subunits and the higher sensitivity of olfactory adenylate cyclase to nonhydrolyzable GTP analogs are consistent with the possible existence of different Gs variants. Signal amplification due to the olfactory G protein may be responsible for the unusual acuity of the sense of smell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignetti E., Cavaggioni A., Pelosi P., Persaud K. C., Sorbi R. T., Tirindelli R. Purification and characterisation of an odorant-binding protein from cow nasal tissue. Eur J Biochem. 1985 Jun 3;149(2):227–231. doi: 10.1111/j.1432-1033.1985.tb08916.x. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Beauchamp G. K., Yamazaki K., Bard J., Thomas L. Chemosensory communication. A new aspect of the major histocompatibility complex and other genes in the mouse. Oncodev Biol Med. 1982;4(1-2):101–116. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
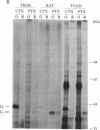
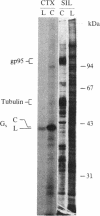
- Chen Z., Lancet D. Membrane proteins unique to vertebrate olfactory cilia: candidates for sensory receptor molecules. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1859–1863. doi: 10.1073/pnas.81.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Pace U., Ronen D., Lancet D. Polypeptide gp95. A unique glycoprotein of olfactory cilia with transmembrane receptor properties. J Biol Chem. 1986 Jan 25;261(3):1299–1305. [PubMed] [Google Scholar]
- Farfel Z., Brothers V. M., Brickman A. S., Conte F., Neer R., Bourne H. R. Pseudohypoparathyroidism: inheritance of deficient receptor-cyclase coupling activity. Proc Natl Acad Sci U S A. 1981 May;78(5):3098–3102. doi: 10.1073/pnas.78.5.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V., Margolis F. L., Getchell M. L. Perireceptor and receptor events in vertebrate olfaction. Prog Neurobiol. 1984;23(4):317–345. doi: 10.1016/0301-0082(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Harris B. A., Robishaw J. D., Mumby S. M., Gilman A. G. Molecular cloning of complementary DNA for the alpha subunit of the G protein that stimulates adenylate cyclase. Science. 1985 Sep 20;229(4719):1274–1277. doi: 10.1126/science.3839937. [DOI] [PubMed] [Google Scholar]
- Henkin R. I. Impairment of olfaction and of the tastes of sour and bitter in pseudohypoparathyroidism. J Clin Endocrinol Metab. 1968 May;28(5):624–628. doi: 10.1210/jcem-28-5-624. [DOI] [PubMed] [Google Scholar]
- Holley A., Mac Leod P. Transduction et codage des informations olfactives chez les vertébrés. J Physiol (Paris) 1977;73(6):725–848. [PubMed] [Google Scholar]
- Kaissling K. E. Chemo-electrical transduction in insect olfactory receptors. Annu Rev Neurosci. 1986;9:121–145. doi: 10.1146/annurev.ne.09.030186.001005. [DOI] [PubMed] [Google Scholar]
- Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984 Mar 25;259(6):3578–3585. [PubMed] [Google Scholar]
- Kurihara K., Koyama N. High activity of adenyl cyclase in olfactory and gustatory organs. Biochem Biophys Res Commun. 1972 Jul 11;48(1):30–34. doi: 10.1016/0006-291x(72)90339-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancet D. Vertebrate olfactory reception. Annu Rev Neurosci. 1986;9:329–355. doi: 10.1146/annurev.ne.09.030186.001553. [DOI] [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa L. M., Hedlund B., Shepherd G. M. Electrophysiological properties of identified cells in the in vitro olfactory epithelium of the tiger salamander. J Neurosci. 1985 Jan;5(1):128–135. doi: 10.1523/JNEUROSCI.05-01-00128.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Trifiletti R. R., Strittmatter S. M., Snyder S. H. Isolation and characterization of an olfactory receptor protein for odorant pyrazines. Proc Natl Acad Sci U S A. 1985 May;82(9):3050–3054. doi: 10.1073/pnas.82.9.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhein L. D., Cagan R. H. Biochemical studies of olfaction: isolation, characterization, and odorant binding activity of cilia from rainbow trout olfactory rosettes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4412–4416. doi: 10.1073/pnas.77.8.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Schleifer L. S., Garrison J. C., Sternweis P. C., Northup J. K., Gilman A. G. The regulatory component of adenylate cyclase from uncoupled S49 lymphoma cells differs in charge from the wild type protein. J Biol Chem. 1980 Apr 10;255(7):2641–2644. [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Smigel M., Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. Mechanisms of guanine nucleotide-mediated regulation of adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:1–18. [PubMed] [Google Scholar]
- Spiegel A. M., Levine M. A., Marx S. J., Aurbach G. D. Pseudohypoparathyroidism: the molecular basis for hormone resistance--a retrospective. N Engl J Med. 1982 Sep 9;307(11):679–681. doi: 10.1056/NEJM198209093071111. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]