Abstract
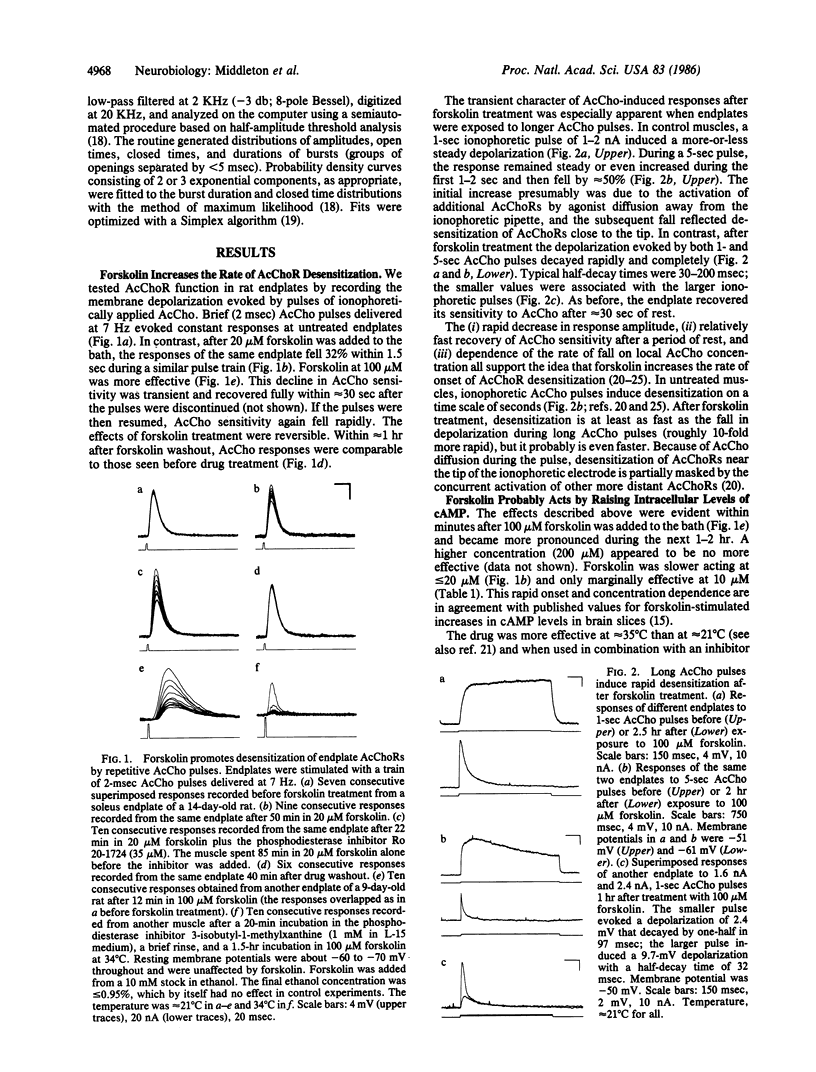
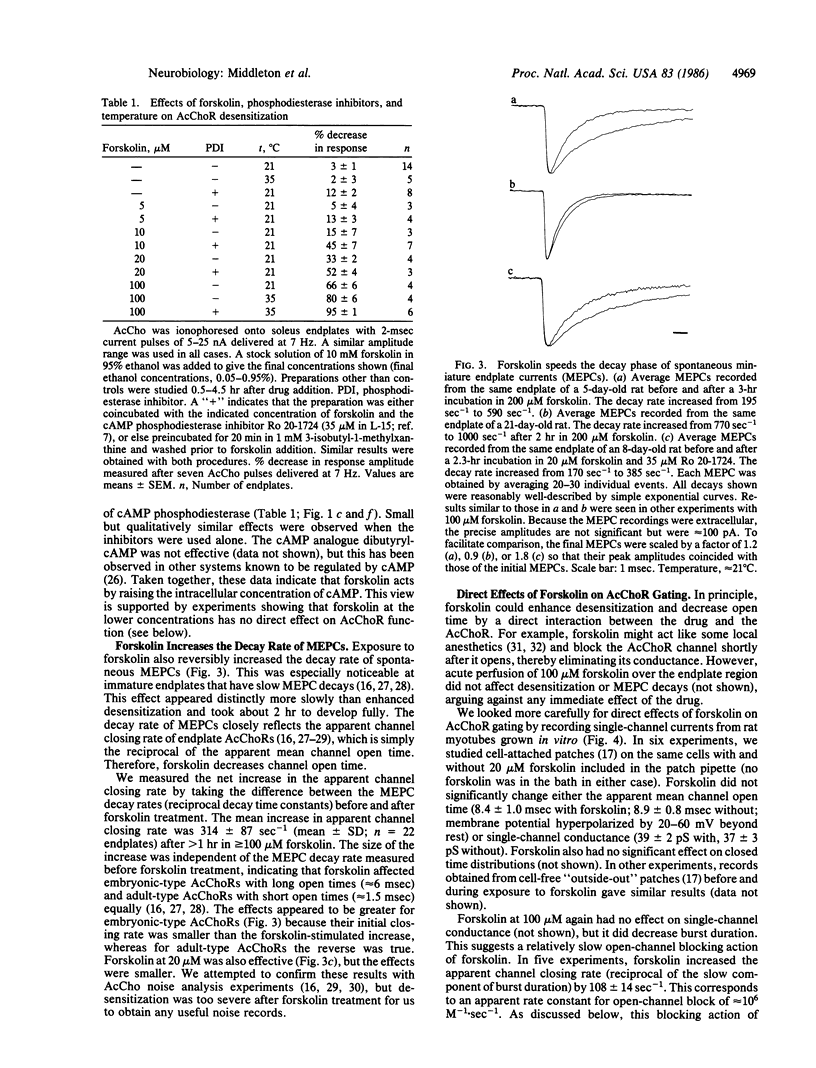
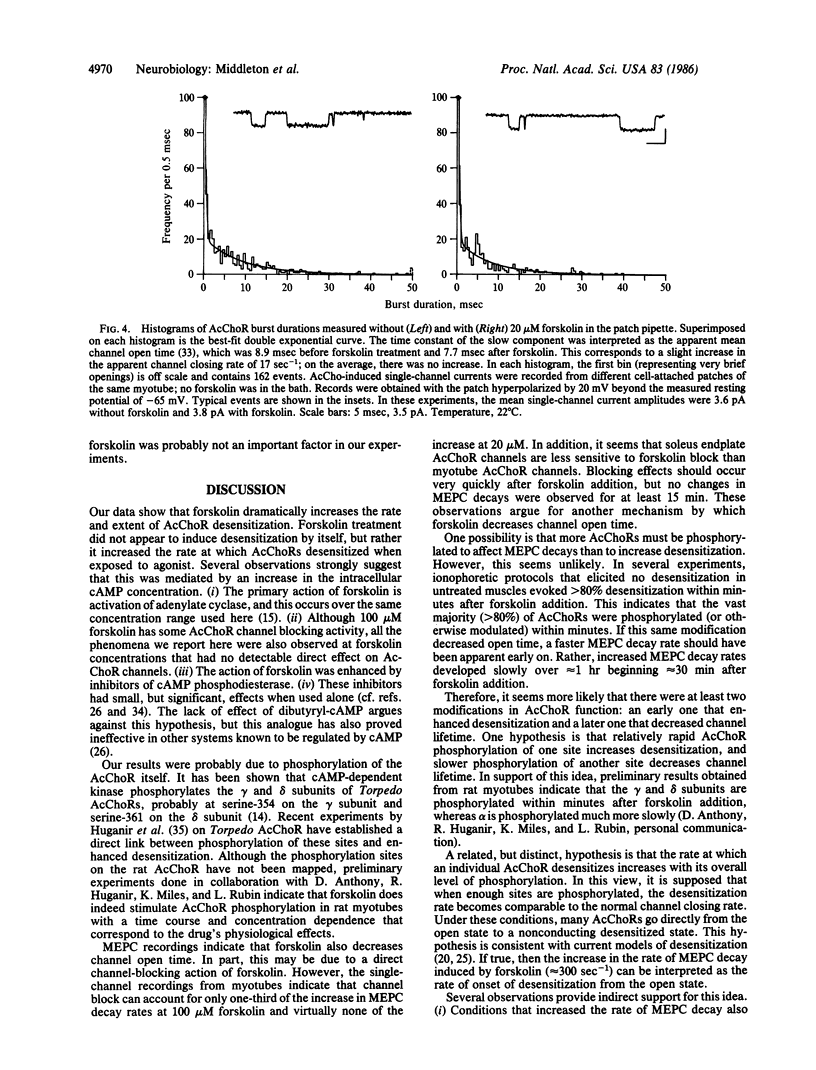
We have studied the function of acetylcholine (AcCho) receptors (AcChoRs) in rat soleus endplates before and after exposing the muscles to forskolin, a potent activator of adenylate cyclase. AcChoR function was tested by recording the membrane depolarization evoked by pulses of ionophoretically applied AcCho. Brief (2 msec) AcCho pulses delivered at 7 Hz evoked constant responses at untreated endplates. In contrast, after 10-100 microM forskolin was added to the bath, responses to similar pulse trains fell by as much as 80% within 1 sec. AcCho sensitivity recovered completely in less than 1 min after the pulses were stopped but fell again when the pulses were resumed. Similarly, longer (1 sec) ionophoretic AcCho pulses evoked roughly constant responses at control endplates, but after forskolin treatment the depolarization fell by one-half within less than 200 msec. These results indicate that forskolin increases the rate at which AcChoRs desensitize when exposed to agonist. Focal extracellular recordings showed that 20-100 microM forskolin also increased the decay rate of miniature endplate currents, indicating that forskolin may decrease AcChoR channel open time. Inhibitors of cAMP phosphodiesterase increased the potency of forskolin. When used alone, these inhibitors had effects similar to those of forskolin but smaller. Patch-clamp experiments indicated that forskolin at 100 microM may also interact with AcChoR channels directly, but at 20 microM this effect is negligible. Therefore, it is likely that the forskolin effects were mediated primarily by increased levels of intracellular cAMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Deshpande S. S., Aracava Y., Alkondon M., Daly J. W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. A study with forskolin and its analogs. FEBS Lett. 1986 Apr 7;199(1):113–120. doi: 10.1016/0014-5793(86)81235-2. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Acosta-Urquidi J., Olds J., Kuzma G., Neary J. T. Protein kinase injection reduces voltage-dependent potassium currents. Science. 1983 Jan 21;219(4582):303–306. doi: 10.1126/science.6294830. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M. D., Huganir R., Greengard P. Protein phosphorylation and neuronal function. J Neurochem. 1985 Jul;45(1):11–23. doi: 10.1111/j.1471-4159.1985.tb05468.x. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2528–2532. doi: 10.1073/pnas.82.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M., Zani B. M. Agents that activate protein kinase C reduce acetylcholine sensitivity in cultured myotubes. J Cell Biol. 1985 Apr;100(4):1339–1342. doi: 10.1083/jcb.100.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald D. A., Williams A., Levitan I. B. Modulation of single Ca2+-dependent K+-channel activity by protein phosphorylation. Nature. 1985 Jun 6;315(6019):503–506. doi: 10.1038/315503a0. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Diamond I. Phosphorylation of acetylcholine receptor by endogenous membrane protein kinase in receptor-enriched membranes of Torpedo californica. Nature. 1977 Jun 9;267(5611):539–540. doi: 10.1038/267539a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1130–1134. doi: 10.1073/pnas.80.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Strumwasser F. A voltage-clamp analysis of currents underlying cyclic AMP-induced membrane modulation in isolated peptidergic neurons of Aplysia. J Neurophysiol. 1984 Aug;52(2):340–349. doi: 10.1152/jn.1984.52.2.340. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. P., Earnest J. P., Young E. F., Choe S., Stroud R. M. The molecular neurobiology of the acetylcholine receptor. Annu Rev Neurosci. 1986;9:383–413. doi: 10.1146/annurev.ne.09.030186.002123. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Brenner H. R. Change in synaptic channel gating during neuromuscular development. Nature. 1978 Nov 23;276(5686):401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster M. J., Camardo J. S., Siegelbaum S. A., Kandel E. R. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. 1985 Jan 31-Feb 6Nature. 313(6001):392–395. doi: 10.1038/313392a0. [DOI] [PubMed] [Google Scholar]
- Strong J. A. Modulation of potassium current kinetics in bag cell neurons of Aplysia by an activator of adenylate cyclase. J Neurosci. 1984 Nov;4(11):2772–2783. doi: 10.1523/JNEUROSCI.04-11-02772.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichberg V. I., Sobel A., Changeux J. P. In vitro phosphorylation of the acetylcholine receptor. Nature. 1977 Jun 9;267(5611):540–542. doi: 10.1038/267540a0. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Drake P. F. The effects of cyclic nucleotide agents on neurons in Aplysia. Brain Res. 1979 Jun 8;168(3):643–647. doi: 10.1016/0006-8993(79)90321-4. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Levitan I. B. Alteration of electrical activity in molluscan neurones by cyclic nucleotides and peptide factors. Nature. 1976 May 6;261(5555):62–64. doi: 10.1038/261062a0. [DOI] [PubMed] [Google Scholar]
- Vicini S., Schuetze S. M. Gating properties of acetylcholine receptors at developing rat endplates. J Neurosci. 1985 Aug;5(8):2212–2224. doi: 10.1523/JNEUROSCI.05-08-02212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



