Abstract
HIV gp41 is a metastable protein whose native conformation is maintained in the form of a heterodimer with gp120. The non-covalently associated gp41/gp120 complex forms a trimer on the virus surface. As gp120 engages with HIV’s receptor, CD4, and coreceptor, CXCR4 or CCR5, gp41 undergoes several conformational changes resulting in fusion between the viral and cellular membranes. Several lipophilic and amphiphilic domains have been shown to be critical in that process. While the obvious function of gp41 in viral entry is well-established its role in cellular membrane fusion and the link with pathogenesis are only now beginning to appear. Recent targeting of gp41 via fusion inhibitors has revealed an important role of this protein not only in viral entry but also in bystander apoptosis and HIV pathogenesis. Studies by our group and others have shown that the phenomenon of gp41-mediated hemifusion initiates apoptosis in bystander cells and correlates with virus pathogenesis. More interestingly, recent clinical evidence suggests that gp41 mutants arising after Enfuvirtide therapy are associated with CD4 cell increase and immunological benefits. This has in turn been correlated to a decrease in bystander apoptosis in our in vitro as well as in vivo assays. Although a great deal of work has been done to unravel HIV-1 gp41-mediated fusion mechanisms, the factors that regulate gp41-mediated fusion versus hemifusion and the mechanism by which hemifusion initiates bystander apoptosis are not fully understood. Further insight into these issues will open new avenues for drug development making gp41 a critical anti-HIV target both for neutralization and virus attenuation.
Envelope glycoprotein structure and function
Env glycoprotein heterotrimer decorates the surface of virion particles as well as infected cells. The primary function of the Env glycoprotein complex is to mediate entry of virus into cells via fusion of viral and cellular membranes in a complex sequence of events. Each monomer is composed of a surface unit, gp120 that facilitates binding of the virus to the receptor, CD4, and a coreceptor, CXCR4/CCR5. The transmembrane subunit, gp41, then mediates the fusion of viral and cellular membranes post receptor/coreceptor binding. The complex sequence of events that are involved in the fusion process (Figure 1) have been elucidated by the use of numerous drugs and peptides that inhibit this process at different steps as well as numerous mutational studies [1]. Generally speaking, binding of the gp120 subunit to CD4 and CXCR4/CCR5 initiates a conformational change in the Env complex that exposes the fusion peptide of gp41 that inserts into the opposing membrane. This is followed by the refolding of the gp41 protein along the coiled coil domains pulling the membranes in close apposition to mediate fusion. Understanding of the fusion process mediated by gp41 has largely been a result of inhibition studies using peptides that mimic N- or C-terminal coiled coil domains and inhibit the formation of intermediate conformations of gp41. While the primary purpose of the Env glycoprotein is to mediate virus entry, its role in HIV pathogenesis is now becoming evident [2, 3].
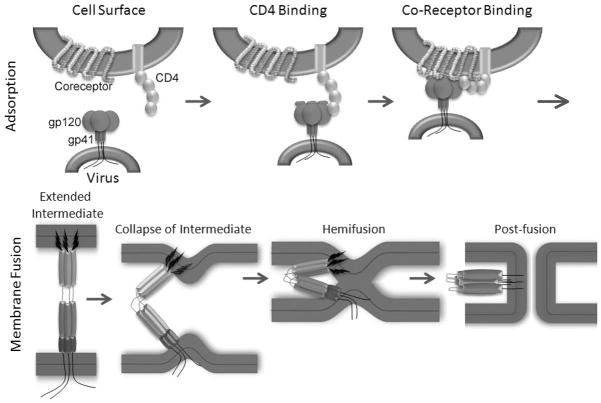
Figure 1. A simplified diagram depicting the process of HIV entry.
Top panel: The envelope protein is a trimer of heterodimers on the virus surface made up of the transmembrane subunit, gp41, embedded in the viral membrane and the globular surface subunit, gp120, non-covalently associated to gp41. Association between gp120 and CD4 causes conformational changes in gp120 that lead to binding to coreceptor.
Bottom panel: Coreceptor binding triggers further concerted conformational changes, including formation of agp41 extended intermediate, that allow gp41 to initiate mixing of the outer bilayer leaflet resulting in formation of the hemifusion state. Finally, the inner leaflets mix resulting in membrane pore formation and injection of the virus core which is concomitant with gp41 six-helix bundle formation.
Envelope Expression and Processing
The HIV envelope proteins are expressed as a precursor protein, gp160, in the endoplasmic reticulum. The precursor then transits the Golgi apparatus where glycosylation takes place. The precursor is cleaved in the trans-Golgi by the cellular protease, furin, into two proteins, gp120 and gp41. These proteins are present on the cell surface as the envelope complex, a mushroom-shaped trimer of heterodimers of gp120 and gp41, incorporated into the virus envelope through the transmembrane region of gp41 as virus particles bud from the cell surface [Adamson et al for detailed review] [4].
Functional Domains of gp41
HIV gp41 can be divided into 3 major domains: 1) the extracellular domain or ectodomain (residues 512–683 by standard HIV-1 HXB2 gp160 numbering), 2) the transmembrane domain (MSD, 683–707) and 3) the cytoplasmic domain (708–856). The major functions of the gp41 protein are mediated by the extracellular domain which can be further subdivided into the following five functional regions: a fusion peptide (FP, 512–534) followed by the N-terminal heptad repeat (NHR/HR1, 542–591), the loop region (593–622) and the C-terminal heptad repeat (CHR/HR2, 623–661) and finally the membrane proximal external region (MPER 662–683) (Figure 2). The NHR can further be subdivided into a pocket-forming domain (PFD) and a heptad repeat sequence (HR). The CHR similar to NHR contains an Heptad repeat region consisting of repeating amino acids which can a form a coiled-coil structure as has been detailed in the atom-level structures of the gp41 ectodomain as described below.
Figure 2. Schematic diagram of different regions of HIV gp41.
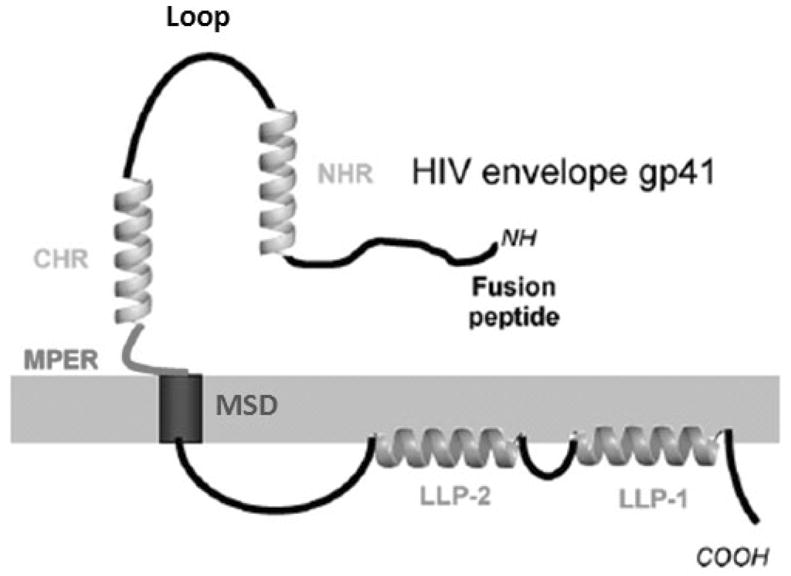
NHR: N Heptad Repeat, CHR: C Heptad Repeat, MPER: Membrane Proximal Ectodomain Region, MSD: Membrane Spanning Domain, LLP1, LLP2: Lentiviral Lytic Peptide 1 and 2.
Structural Information on gp41
Several cryo-electron tomography studies have produced alternative structures of the gp41 portion of the envelope complex in the native state [5–8]. Some of these studies suggest that the native structure is that of a mushroom shape with a stalk-like structure protruding from the viral membrane [5, 7]. The stalk-structure presumably represents the electron density of gp41 which is then covered by a globular three-lobed structure representing gp120 electron density [5, 7]. Whereas some of the studies have suggested that the gp41 electron density is in a tripod-shaped structure with space between the three legs proximal to the membrane [6, 8]. An interesting study was published recently suggesting that the reason for these apparent discrepancies could be that there are strain-dependent variations in the quaternary structure of the envelope complex on the surface [9].
At the atomic level, three important crystal structures were reported in 1997 [10–12]. These three structures are each of the central core of what is described as the six-helix bundle of HIV gp41. This structure is presumed to be the post-fusion conformation of gp41 because of its high thermostability [13, 14]. In addition, an NMR structure of the Simian Immunodeficiency Virus (SIV) gp41 was reported that includes the central immunogenic loop region and which extends further into the helical regions toward the N- and C- termini of the protein [15]. All of the atomic-level (x-ray and NMR) structures to date are limited to the post-fusion conformation of gp41.
Lipophilic and amphiphilic domains of gp41
The gp41 transmembrane protein plays a critical role in the complex fusion process. The lipophillic and amphiphilic nature of multiple gp41 regions along with the complex conformational changes that conclude the fusion process have prompted a number of mutational and inhibition studies to better understand this process.
Fusion peptide
gp41 is a class 1 fusion protein that results from the cleavage by furin of a longer precursor, gp160. Adjacent to the processing site resides a long hydrophobic stretch of amino acids that forms the N-terminus of gp41 after cleavage. Mutations performed within this domain like polar substitutions of hydrophobic amino acids [16], or deletions [17] result in a dramatic decrease in the ability of the virus to fuse with target cells. Multiple studies have therefore aimed at understanding the unique features of this 23 AA long hydrophobic portion of gp41 termed the “fusion peptide.” Although experimentally not proven so far, the fusion peptide is believed to insert into the target cell membrane during the conformational reorganization of gp41. At this stage, gp41 would be in an extended conformation physically linking the viral membrane and the target membrane. Studies using the complete gp41 protein demonstrate the importance of the hydrophobic nature of this domain and the importance of specific amino acids notably within the highly conserved FLGFLG sequence [18]. However, the overall hydrophobicity of gp41 makes it particularly difficult to handle as a whole protein [19]. Most of the functional and structural studies performed with synthesized fusion peptides and model membranes have shown that this portion of gp41, on its own, has the ability to destabilize liposomes, induce lipid mixing, and leakage of vesicular contents [20]. A V2E mutation, in the fusion peptide has been shown within the context of the whole envelope to abrogate viral fusion [16]. The same substitution was associated, at the peptide level, to an inability to induce lipid mixing and vesicular leakage [21] supporting the notion that synthetic peptides might be able to give insights into the mechanism of action of the entire protein. Great attention has therefore been given to decipher the structural elements responsible for the activity of the fusion peptide. The FP has the ability to bind to lipidic membranes and its fusogenic activity has been related to its orientation within the bilayer [22]. This has given rise to the concept of tilted peptides [23] whereby some proteins would rely on peptides that insert into membranes at an oblique angle close to 45° to facilitate their function. This angle can considerably disrupt the alignment of phospholipid alkyl chains resulting in membrane destabilization [24]. Such peptides would be shielded under normal conditions to prevent their destabilizing effect on membranes and exposed at specific times, like during the fusion reaction. To support this concept, they would need to acquire a helical conformation. The conformation of gp41 fusion peptide is highly plastic as both α-helical [25] as well as β-strand [26] were observed. This conformational transition seems to be highly dependent on several factors such as the lipidic composition of the membrane of insertion, the peptide concentration within the membrane and the presence of cholesterol [27] or calcium [28]. The presence of cholesterol in the membrane specifically seems to favor the β-sheet conformation [27]. Lower peptide concentrations on the other hand favors α helical conformations whereas the β-sheet conformation becomes more preponderant at higher concentrations [29]. This β-sheet conformation can lead to the formation of fibrils giving to gp41 fusion peptide some of the properties of amyloid peptides [30]. Functional assays indicate however that small aggregates would be more fusogenic than either monomers or large aggregates. At similar concentrations, fusion peptide presented as trimers can induce a 15-fold increase in fusion as compared to their monomer form and this showed a strong correlation with the depth of insertion [26, 31].
While the study of synthetic fusion peptides provides a lot of insight into the function of gp41, and the analysis of their membrane action can yield promising drugs [32] the fusion peptide acts within the context of a larger protein that is likely to modulate its effects. Within the 23 amino acid long fusion peptide domain of gp41, the first 16 N-terminal residues are the most hydrophobic and are by themselves able to destabilize and induce lipid mixing of liposomes. The following 17 amino acids are more hydrophilic but their palmitoylation can facilitate their interaction with membranes and allow the induction of membrane fusion independently of the presence of the hydrophobic stretch of the fusion peptide. Put together, those two components acquire an enhanced fusogenic capability and a propensity of self-association not present within the individual domains [33].
N Heptad (NHR) and Loop region
Adjacent to the fusion peptide domain (Figure 2) is located another highly conserved hydrophobic region of gp41 ectodomain, the NHR domain. The NHR domain of gp41 is known to form a coiled coil structure as it is part of the ectodomain structure that has been solved. Peptides derived from this region have been shown to be membranotropic and are able to induce vesicle destabilization, leakage, lipid mixing and aggregation [34]. Owing to their positive charge, this effect is observed in negatively charged bilayers. In such a lipidic environment, a significant shift in the conformation of those peptides from α-helical to β-strand has been observed [35]. The outer leaflet of cells does not contain negatively charged phospholipids that are actively maintained in the inner cytoplasmic side of the lipidic bilayer [36, 37] Transient exposure of phosphatidyl serine PS has been observed in some extreme conditions [38, 39] and whether this can take place upon the exposure and potential insertion of the fusion peptides remains to be determined. Nevertheless, the activity of the fusion peptide that was shown to be amplified by the presence of the adjacent polar amino acids [33] is considerably increased by the presence of the full NHR domain [40].
The portion immediately adjacent to the NHR domain corresponds to the loop region (Figure 2). Although little information is available on the relevance of this domain in the fusion process, mutational analysis has shown its role in the association of gp41 with gp120 [14, 41]. A peptide screen of the entire ectodomain of gp41 has shown, that this portion presented a strong interaction with membranes [42]. Further analysis of peptides from this region revealed a high affinity binding with negatively charged membranes similar to the positively charged NHR peptides. These peptides were also shown to be able to induce membrane destabilization and vesicle leakage possibly due to aggregation within membranes determined by conformational studies [34]. This region of gp41 therefore, seems, to share some lipophilic similarity with the adjacent NHR domain.
Membrane Proximal External Region (MPER)
Adjacent to the loop domain is the C-heptad repeat (CHR) domain that terminates a long stretch of hydrophobic segments, followed by the MPER domain. Functional studies have shown that the MPER region of gp41 is critical for viral entry. Mutations within this region can prevent viral entry or block it at the level of pore expansion [43]. A recent analysis provided evidence that MPER was critical for envelope incorporation into the virion as well as functionally for its ability to perturb membranes explaining its high level of conservation.[44] Indeed, as is the case for the above mentioned gp41 domains, this tryptophan enriched region is highly membranotropic. MPER can interact with membranes adopting an α-helical conformation and induce vesicle leakage as well as lipid mixing [45]. This domain has been further divided into two regions that harbor different membrane destabilization phenotypes [46]. While many studies support an α-helical conformation for this domain, other conformations have also been reported. Interestingly this region is recognized by broadly neutralizing antibodies. Studies of the antibody epitope interaction reveal the presence of β-strands as well as S shaped structure composed of perpendicular helical turns in addition to the α-helical structure. Overall, these studies gave rise to the notion that conformational switches within this region could be associated with its function in the HIV entry process [47].
Membrane Spanning Domain (MSD)
Although hydrophobicity studies indicate 25 amino acids as the potential membrane spanning domain of gp41, its precise boundaries have not been clearly defined. This portion of gp41 has been modeled to span the membrane bilayer as a single span of α-helix providing anchoring of the protein to the membrane. A function beyond anchoring has been suggested by the inability of GPI-anchored mutants to induce fusion.[48, 49] The precise nature of this involvement is not clear as the fusion capability was preserved by the substitution of the entire spanning domain of gp41 by the one from CD22 [50]. However the fusion capability was lost when the transmembrane domain of glycophorin A, VSVG [51] or HA [52] was used suggesting a complex interaction between the MSD and the extracellular domains of gp41. Detailed mutational studies indicate that the length of the transmembrane segment is critical for proper viral function beyond anchoring and that within this domain resides a core region of 12 amino acids, the composition of which influences the ability of gp41 to undergo proper fusion [53, 54].
Lentiviral Lytic Peptides (LLP)
Gp41 contains an unusually long cytoplasmic domain that has been associated with a wide variety of functions. It contains highly conserved lentiviral lytic peptides (LLPs) that are amphipathic α-helical domains that have been shown to be inserted into the viral membrane in the context of intact virus [55]. The precise function of these peptides is not understood but they seem to be involved in an “inside-out” mechanism of regulation of the envelope function. Indeed, truncation of the LLP2 peptide has been shown to result in increased fusion efficiency while leading to the enhanced exposure of gp120 conformational epitopes [56]. The nature of the mechanism of regulation is poorly understood. Based on epitope exposure, the group of Dimmock has hypothesized that the cytoplasmic tail of gp41 is not completely internal [57]. According to this notion, part of the C-terminus of the cytoplasmic tail would therefore be able to directly interact with the ectodomain of the protein and this would occur in a dynamic fashion with transient epitope exposure [58, 59].
The composition of gp41 is rich in domains able to interact with lipidic membranes. They have all been shown to be critical for the proper mediation of the fusion event by gp41 and are presumably essential for the hemifusion phenotype. Their individual study as synthetic peptides has allowed the evaluation of their ability, on their own, to destabilize membranes, induce vesicle leakage and actuate lipid redistribution. Care must be taken, however, to extrapolate their function within the context of gp41 as their activity will be constrained by the structure of the remainder of the protein and the lipid environment along the fusion process [60, 61]. Overall, structural analyses have shown a great plasticity of the different domains of gp41 that is likely essential for its function as a fusion protein. The complex interplay between the different components of the protein along the fusion process will have to be further understood notably through kinetic structural analysis in the context of the entire protein [62–65]. Indeed, several peptides can present synergism in their action (NHR with FP [40]) while others can have antagonist effects (CHR with FP, NHR and MPER [66], LLP2 [56]). Heterologous and homologous interactions taking place between the different domains of gp41 have been evidenced by the inhibitory capacity of peptides mimicking gp41 sequence from the FP domain [32, 67, 68] the NHR domain [69] the CHR domain [70] [69] and the MPER domain [71]. All of the lipophilic domains of gp41, considering their high conservation across clades, present an interesting avenue for the development of novel therapeutics.
HIV gp41 inhibition: neutralizing antibodies, peptides, and small molecule inhibitors
Neutralizing antibodies
The highly conserved nature of gp41 makes it an excellent target for drug development. In fact the earliest evidence of the conserved nature of gp41 and its potential use as a vaccine/drug target came from broadly cross reacting neutralizing antibodies (nAbs). Two of these nAbs, 2F5 and 4E10, interact with gp41 to inhibit the fusion step of viral entry [72]. Both antibodies bind to the MPER region which is positioned in sequence after the CHR but which precedes the transmembrane region. This region is hydrophobic, highly conserved and critical for fusion. However, when attempts to re-elicit this response in vivo have been made, the antibodies have met with limited success in preventing infection and lowering viral loads [73, 74]. This limited effectiveness is illustrative of how well-protected HIV envelope is to immune response. Indeed, most of the epitopes to which the envelope antibodies are directed are protected in the mature virion. We have previously reported that escape from neutralization occurs concomitantly with resistance to the peptide inhibitors that bind to the NHR.[64] Addition of peptide inhibitors, however, did not counteract the loss of the 2F5/4E10 binding, suggesting that changes in exposure of these two regions occur concomitantly but independently. Attempts to re-elicit antibody production by vaccination with 2F5/4E10 epitopes have produced antibodies that react with the immunogen but not with the virus [75]. Interestingly the epitope recognized by 2F5 and 4E10 is a lipid peptide complex that has been reported to mimic cardiolipin in humans [76]. Owing to their autoantigen binding phenotype, these antibodies were rarely seen in patients and have been even harder to generate using conventional immunogens or peptides as vaccine strategy [77, 78]. Additional studies are needed to gain a better understanding of the native and intermediate structures of gp41.
Peptide inhibitors
In the early 1990s, researchers discovered that peptides derived from the N-heptad repeat region (NHR) or from the C-heptad region (CHR) of HIV gp41 inhibit viral entry in cell culture [[79] for review]. There are two prominent peptides, C34 and T20, which are from the same region of the CHR but are shifted by just ten amino acids in sequence in relation to each other. The T20 sequence is located more proximal to the viral membrane than the C34 sequence. The development of these two peptides has followed different pathways and evidence indicates that their mechanism of action may be different as well [80]. T20 progressed into pre-clinical and clinical trials and is employed in the clinic under the name Fuzeon or Enfuvirtide. Significant problems with T20 therapy exist that appear to be inherent in peptide therapies in general such as lack of oral bioavailability, rapid renal clearance, a short half-life of two hours, injection site reaction and high cost. At this time, T20 therapy is restricted to a salvage therapy for treatment-experienced patients. Peptide C34, on the other hand, has remained a laboratory reagent and its use is widespread among the world’s laboratories. A notable report provided evidence that the two peptides work by different mechanisms [80]. T20 binds to a variety of binding sites and C34 binding is far more specific. C34 binds to the pocket-forming domain and the HR sequence of the NHR, inhibiting six-helix bundle formation and fusion. T20, on the other hand, lacking the pocket binding domain, binds to the HR and also to the lipid membrane to inhibit fusion. The fact that resistance to T20 can arise quite rapidly both in vitro as well as in vivo [81–85] has prompted the development of the next generation of peptide inhibitors. The second generation peptides developed by Trimeris Inc. include T1249 and T1144 based on activity against T20 resistant peptides [86]. Recently Dwyer et al have used a unique strategy involving structural homology to gp41 rather than sequence homology to design the third generation of anti HIV peptide T2635 with increased activity against T20 resistant viruses [87]. Sifuvirtide is another peptide currently in clinical development for anti HIV therapy [88, 89]. In an effort to improve upon the efficacy of peptide inhibitors, we have developed conjugation methods by which we can target specific amino acids on gp41 and covalently link peptide inhibitors in order to permanently trap the gp41 intermediate [90]. The linking of anti HIV peptides to cholesterol [91] or albumin [92] are alternative strategies that have been demonstrated to greatly enhance activity and half life of gp41 targeted anti HIV peptides. Other advances being made in targeting the gp41 intermediate include a variation of peptides that combine elements from both C34 and T20 [[93] for detailed review]. Sequence optimization for enhanced solubility and stability is another goal as is construction of D-amino acid peptides in order to prevent proteolytic degradation. Phage display has been used successfully to identify and further develop cyclic D-peptides that have inhibitory activity against a hydrophobic pocket that is transiently exposed on gp41 during fusion [94, 95]. The most recent advancement in this type of peptide inhibitor has been identification of a D-peptide with potency adequate for clinical development and which also showed lowered development of resistance [96]. The identification of inhibitory D-peptides targeted to the gp41 pocket has helped to validate this region as a promising drug target. It is interesting to note that short gp41 N-terminal peptides which are membrane anchored but lack the critical pocket domain are also inhibitory both in viral entry assays and in cell-cell fusion [97]. Recombinant proteins are also being constructed in order to potentially lower production costs. A bacterially expressed fusion inhibitor was constructed combining the sequences of C34 and T-20 and has proven to be effective in vitro and has demonstrated promising results in microbicide development studies.[98, 99] Another recombinant inhibitor was constructed using peptides derived from the N-terminal heptad repeat. N-terminal peptides are usually not considered to be good candidates because of their propensity for aggregation instead of formation of discrete coiled-coil trimers [100]. The novel protein is made up of N-peptides that have been fused to the T4 fibritin trimerization domain, Foldon. This protein was able to form a stable six-helix bundle with the addition of C-peptide and showed potent inhibition against a broad spectrum of HIV strains in vitro [100].
Small Molecule Inhibitors
Gp41 has also been a target for development of numerous non peptide small molecule inhibitors in order to circumvent the problems of peptide therapies such as the short half-life and lack of oral bioavailability. Structural analysis of gp41 core revealed that a deep hydrophobic pocket (Trp, Trp-Ile pocket) is occupied by highly conserved W628, W631 and I635 residues from the CHR making a potential drug target.[101] High throughput screening methods based on computer modeling of the hydrophobic pocket combined with biochemical assays based on disruption of NHR and CHR interaction have been developed to facilitate the discovery of inhibitors of gp41 [102]. Some of the non peptide inhibitors identified by these approaches include metabolic product of the cell viability dye XTT formazan [103] and ADSJ-1 [104], Benziamide 1 series compounds [105], N substituted pyrrole derivatives NB-2 and NB-64 [106] and N-carboxyphenylpyrrole [107] derivatives that bind to the conserved Trp-Trp-Ile domain and inhibit gp41 mediated fusion. High throughput screening methods have also identified a number of gp41 binding compounds notably 5M041 with activity against a wide range of HIV isolates [108]. More recently using a competitive fluorescence inhibition assay [109], Zhou et al have designed indole compounds with anti HIV activity with IC50 as low as 1μM [110]. While the inhibitory concentration IC50 of most of these is in the μM range and hence need improvement, the advancements in high throughput assays in combination with molecular modeling should help further development of small molecule inhibitors of gp41.
Role in HIV gp41 in apoptosis
The mechanism via which HIV infections lead to a progressive depletion of CD4 cell and AIDS development remains highly debated. The fact that natural infections of the SIV virus in the wild in Sooty Managbeys (SM) and African Green Monkeys (AGM) does not lead to AIDS development suggests that the virus infection per se does not cause depletion of CD4 cells [111–114]. This has led many researchers to believe that the progressive depletion of CD4+ T cells in HIV infection may be attributed to bystander apoptosis induction via the Env glycoprotein.[3] This hypothesis stems from the fact that 1) the number of infected cell far exceeds the number of apoptotic cells in HIV infection. 2) The cell death is restricted to CD4 cells and as Env binds CD4 it is natural that it plays a role in this process. 3) Env glycoprotein is expressed on the surface of infected cells and is a probable candidate for induction of apoptosis in neighboring bystander cells. This was evident from studies by Finkel. et al., in lymph node sections from HIV infected patients whereby apoptotic cells were largely uninfected and in close proximity to productively infected cells [115]. While the argument in favor of Env glycoprotein is strong, the mechanism via which Env glycoprotein induces apoptosis also remains highly controversial.[2] Early studies suggested that the binding of Env glycoprotein to CD4 receptor or the cognate co receptor CXCR4/CCR5 may initiate aberrant signaling leading to apoptosis [116, 117]. This is supported by observations that blocking CD4 and/or correceptor interaction with the Env glycoprotein inhibited Env-mediated bystander apoptosis in in vitro assays [118–120]. While these findings are strong indicators that the gp120 subunit interactions with CD4 and CXCR4 are essential for apoptosis, it does not provide a mechanism for apoptosis induction. Signaling pathway inhibition downstream of CD4 as well as CXCR4 failed to inhibit the apoptosis induction via gp120 binding to CD4 and CXCR4 [121–123]. This suggests that while binding of the Env glycoprotein to the receptor and coreceptor is absolutely critical to apoptosis induction these interactions are not sufficient for the process. This prompted the search for downstream events to gp120 binding that may play a role in bystander apoptosis. The necessary break came with the development of peptide inhibitors of gp41-mediated fusion process like the C34 and T20 peptides [69]. Using these inhibitors, it was demonstrated that HIV Env glycoprotein mediated apoptosis could be inhibited at the level of gp41 [124, 125]. This suggested that although gp120 binding was essential, the downstream events mediated by gp41 were critical for bystander apoptosis induction.
Evidence for the role of Env fusion activity in HIV pathogenesis has been supported by other studies. Clinical studies have classified viruses as being either syncytia-inducing (SI) or non-syncytia-inducing (NSI) based on syncytia formation in MT-2 cell culture [126]. The likelihood of finding a syncytia-inducing phenotype in patients has been associated with poor prognosis and progression to AIDS [127, 128]. Although broadly speaking SI viruses have been known to be CXCR4 (X4) utilizing and NSI viruses CCR5 (R5) utilizing [129], the requirement of a coreceptor switch from R5 to X4 in AIDS progression is not essential.[130] In recent studies it has been found that the R5 viruses that are associated with progression to AIDS are more fusogenic than pre AIDS viruses, once again indicating a strong correlation with gp41 function [131, 132].
Experimentally the role of the fusion process in AIDS development in vivo also came from studies by Reimann, et al. [133] Using the SHIV 89.6 virus containing the HIV 89.6 env, rev, tat and nef regions in an SIV backbone, they demonstrated that the passage of SHIV 89.6 in monkey resulted in development of a pathogenic virus termed SHIV 89.6P [134, 135]. The enhanced pathogenesis was mapped to the Env glycoprotein and an increased fusion activity phenotype was associated with disease [136, 137]. While membrane fusion activity was associated with the phenomenon of increased pathogenesis of SHIV 89.6P it remained unclear whether this was due to autofusion in infected cells or apoptosis induction in bystander cells.
Role of gp41 fusion/hemifusion in bystander apoptosis
Although the role of gp41 in bystander apoptosis is evident, the mechanism via which this process is regulated remained unknown. Ferri, et al., have extensively studied the events post gp41-mediated fusion of cells. They found that syncytia formed due to Env/gp41-mediated fusion are short lived in culture and usually die by 72 hours [138–140]. The signaling pathway involved in this process has been shown to involve p53, MAPK and mTOR [141, 142]. More recently the accumulation of oncosuppressor proteins, ataxia telangiectasia mutated (ATM) and promyelomonocytic leukemia (PML) protein, in the nuclei has been associated with apoptosis in syncytia formed as a result of HIV Env mediated fusion [143, 144]. While these studies clearly define the fate of syncytia arising during HIV infection and explain the role of gp41 in bystander apoptosis to some extent; syncytia formation in vivo is probably quite limited. Furthermore, a number of investigations suggest that the majority of cells undergoing apoptosis in Env coculture experiments are single cells. Even in ex vivo thymic cultures it was demonstrated that apoptosis of bystander cells required the function of gp41 and Env fusion activity [145]. Blanco, et al., used a dye transfer assay to determine the mechanism of bystander apoptosis induction by HIV gp41. When Env expressing cells labeled with either a lipophillic membrane dye or a cytoplasmic dye were cocultured with target cells; apoptosis was seen in cells taking up the lipophilic dye suggesting a hemifusion phenotype. This gave rise to the hypothesis that hemifusion mediated by gp41 initiates apoptosis in bystander cells. The process of hemifusion mediated by viral Env proteins is not rare and most likely an intermediate in the fusion process (Figure 1). A GPI-anchored Influenza virus HA protein has been demonstrated to be restricted at the hemifusion step [146]. This state is generated when only the outer leaflets of the membranes mix followed by separation of cells. [reviewed by Chernomordik et al [147]].
While dye transfer assays supported the notion that hemifusion correlates with bystander apoptosis induction direct evidence supporting this hypothesis was missing. Studies by Alizon, et al., [148] had demonstrated that certain mutations in the loop region of HIV Env are restricted at the hemifusion state. Using this information we asked whether a hemifusion restricted Env glycoprotein would be capable of inducing bystander apoptosis. Interestingly, we found that the D589L mutant in the loop region was restricted at the hemifusion step and was capable of causing bystander apoptosis similar to WT in the absence of syncytia formation [149]. This study provided the first direct evidence that hemifusion alone was sufficient for apoptosis induction in the absence of syncytia formation. Our studies have revealed that apoptosis mediated by gp41 is in fact hemifusion dependent and involves early caspase 3 activation followed by mitochondrial depolarization [150]. However, the events that lead from hemifusion at the plasma membrane to caspase activation are uncertain and an area open to further investigation.
Enfuvirtide resistance and virus attenuation
The evidence that HIV fusion activity regulates bystander apoptosis and pathogenesis gives rise to the idea that altering fusion activity or targeting gp41 could alter HIV pathogenesis. This is evident from our mutational studies of HIV gp41 whereby gp41 mutants with reduced cell to cell fusion activity are also reduced in bystander apoptosis induction [149]. Our studies include a G36D mutation in the highly conserved GIV domain of gp41 that is the hot spot for virus resistance against Enfuvirtide (T20), the peptide inhibitor of gp41 mediated fusion. Alongside these studies several clinical reports show that in some patients receiving Enfuvirtide therapy, the CD4 counts keep increasing even after virological failure. Analysis of the resistance mutations in these patients revealed that a mutation at the V38 position, specifically V38A/E, was associated with this increase [81, 82]. These findings were confirmed in retrospective study by Trimeris, Inc. including a large population of samples [83]. Taken together these studies give rise to the hypothesis that not only is HIV pathogenesis related to gp41 fusion activity but it might be possible to attenuate the virus by targeting certain regions of gp41 like the GIV domain. We undertook an in vitro investigation of the resistant mutations seen in patients with immunological benefits and found that in fact a virus with V38E mutation was severely restricted in bystander apoptosis induction while replicating better than WT in CD4+ T cell line [151]. Recently we have studied the lack of bystander apoptosis inducing phenomenon of the V38E mutant in humanized mice [152]. We find that in fact this single amino acid change can markedly reduce both bystander apoptosis and CD4 decline in the humanized mouse model corroborating our in vitro findings. These studies validate our hypothesis and open new areas of investigation looking at how to manage patients on Enfuvirtide therapy as well as developing new inhibitors of gp41 that would select for even weaker HIV gp41 mutants.
Future directions for drug development and attenuation
Studies on HIV gp41 structure and function have come a long way since the identification of coiled coil domains in the protein and targeting of gp41 by peptide inhibitors. However, the exact mechanistic link between hemifusion and the apoptotic processes within bystander cells is still missing. Further research in this area should be directed towards the elucidation of the primary factors responsible for apoptosis initiation following the interactions between Env-expressing cells and target cells. Examination of events taking place during the formation of the T cell synapse [153–155] may yield further insights into this phenomenon. The interactions of gp41 segments with lipids need to be further investigated in the context of the entire protein to better understand their role in the mode of action of gp41 and allow the development of potent inhibitors targeting those regions. On the other hand, inhibitors of gp41 six-helix bundle formation have already been very successful clinically. It is evident from clinical use of Enfuvirtide that resistance to this group of drugs is bound to occur. However, the understanding of HIV gp41 mediated bystander apoptosis opens new avenues for drug development. For one, it is essential to determine the resistance pattern of mutants arising during Enfivurtide therapy for most patients. An increase in CD4 counts after virological failure may suggest beneficial mutants and could serve as a marker for virus attenuation. New generation gp41 inhibitors should therefore be evaluated for resistance pattern as well as the Env fusogenicity of resistant mutations before use. Some of the new strategies being employed for the third generation fusion inhibitors are the rational design of peptide inhibitors based on structure rather than sequence of the heptad repeats. These new generation inhibitors, like T1144, T2365 and sifuvirtide [88, 89], are more potent and less prone to resistance development than the previous generation. Whether the resistance pattern with these new generation inhibitors is similar to Enfuvirtide and would it result in virus attenuation is a matter of further investigation.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Gallo S, Finnegan C, Viard M, Raviv Y, Dimitrov A, Rawat S, Puri A, Durell S, Blumenthal R. The HIV Env-mediated fusion reaction. Biochim Biophys Acta. 2003;1614:36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 2.Perfettini J, Castedo M, Roumier T, Andreau K, Nardacci R, Piacentini M, Kroemer G. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005;12(Suppl 1):916–23. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- 3.Garg H, Blumenthal R. Role of HIV Gp41 mediated fusion/hemifusion in bystander apoptosis. Cell Mol Life Sci. 2008;65:3134–44. doi: 10.1007/s00018-008-8147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamson CS, Freed EO. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv Pharmacol. 2007;55:347–87. doi: 10.1016/S1054-3589(07)55010-6. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti G, Briggs JA, Grunewald K, Sattentau QJ, Fuller SD. Cryo-electron tomographic structure of an immunodeficiency virus envelope complex in situ. PLoS Pathog. 2006;2:e83. doi: 10.1371/journal.ppat.0020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu P, Liu J, Bess J, Jr, Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. Distribution three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441:847–52. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008;455:109–13. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu SR, Loving R, Lindqvist B, Hebert H, Koeck PJ, Sjoberg M, Garoff H. Single-particle cryoelectron microscopy analysis reveals the HIV-1 spike as a tripod structure. Proc Natl Acad Sci U S A. 2010;107:18844–9. doi: 10.1073/pnas.1007227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White TA, Bartesaghi A, Borgnia MJ, Meyerson JR, de la Cruz MJ, Bess JW, Nandwani R, Hoxie JA, Lifson JD, Milne JL, Subramaniam S. Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure. PLoS Pathog. 2010;6:e1001249. doi: 10.1371/journal.ppat.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–73. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 11.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci U S A. 1997;94:12303–8. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–30. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 13.Krell T, Greco F, Engel O, Dubayle J, Kennel A, Charloteaux B, Brasseur R, Chevalier M, Sodoyer R, El Habib R. HIV-1 gp41 and gp160 are hyperthermostable proteins in a mesophilic environment. Characterization of gp41 mutants. Eur J Biochem. 2004;271:1566–79. doi: 10.1111/j.1432-1033.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs A, Simon C, Caffrey M. Thermostability of the HIV gp41 wild-type and loop mutations. Protein Pept Lett. 2006;13:477–80. doi: 10.2174/092986606776819510. [DOI] [PubMed] [Google Scholar]
- 15.Caffrey M, Cai M, Kaufman J, Stahl SJ, Wingfield PT, Covell DG, Gronenborn AM, Clore GM. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 1998;17:4572–84. doi: 10.1093/emboj/17.16.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freed EO, Myers DJ, Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990;87:4650–4. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaal H, Klein M, Gehrmann P, Adams O, Scheid A. Requirement of N-terminal amino acid residues of gp41 for human immunodeficiency virus type 1-mediated cell fusion. J Virol. 1995;69:3308–14. doi: 10.1128/jvi.69.6.3308-3314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delahunty MD, Rhee I, Freed EO, Bonifacino JS. Mutational analysis of the fusion peptide of the human immunodeficiency virus type 1: identification of critical glycine residues. Virology. 1996;218:94–102. doi: 10.1006/viro.1996.0169. [DOI] [PubMed] [Google Scholar]
- 19.Viard M, Blumenthal R, Raviv Y. Improved separation of integral membrane proteins by continuous elution electrophoresis with simultaneous detergent exchange: application to the purification of the fusion protein of the human immunodeficiency virus type 1. Electrophoresis. 2002;23:1659–66. doi: 10.1002/1522-2683(200206)23:11<1659::AID-ELPS1659>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Rafalski M, Lear JD, DeGrado WF. Phospholipid interactions of synthetic peptides representing the N-terminus of HIV gp41. Biochemistry. 1990;29:7917–22. doi: 10.1021/bi00486a020. [DOI] [PubMed] [Google Scholar]
- 21.Pereira FB, Goni FM, Muga A, Nieva JL. Permeabilization and fusion of uncharged lipid vesicles induced by the HIV-1 fusion peptide adopting an extended conformation: dose and sequence effects. Biophys J. 1997;73:1977–86. doi: 10.1016/S0006-3495(97)78228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin I, Schaal H, Scheid A, Ruysschaert JM. Lipid membrane fusion induced by the human immunodeficiency virus type 1 gp41 N-terminal extremity is determined by its orientation in the lipid bilayer. J Virol. 1996;70:298–304. doi: 10.1128/jvi.70.1.298-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasseur R. Tilted peptides: a motif for membrane destabilization (hypothesis) Mol Membr Biol. 2000;17:31–40. doi: 10.1080/096876800294461. [DOI] [PubMed] [Google Scholar]
- 24.Epand RF, Martin I, Ruysschaert JM, Epand RM. Membrane orientation of the SIV fusion peptide determines its effect on bilayer stability and ability to promote membrane fusion. Biochem Biophys Res Commun. 1994;205:1938–43. doi: 10.1006/bbrc.1994.2897. [DOI] [PubMed] [Google Scholar]
- 25.Jaroniec CP, Kaufman JD, Stahl SJ, Viard M, Blumenthal R, Wingfield PT, Bax A. Structure and dynamics of micelle-associated human immunodeficiency virus gp41 fusion domain. Biochemistry. 2005;44:16167–80. doi: 10.1021/bi051672a. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Gabrys CM, Weliky DP. Solid-state nuclear magnetic resonance evidence for an extended beta strand conformation of the membrane-bound HIV-1 fusion peptide. Biochemistry. 2001;40:8126–37. doi: 10.1021/bi0100283. [DOI] [PubMed] [Google Scholar]
- 27.Wasniewski CM, Parkanzky PD, Bodner ML, Weliky DP. Solid-state nuclear magnetic resonance studies of HIV and influenza fusion peptide orientations in membrane bilayers using stacked glass plate samples. Chem Phys Lipids. 2004;132:89–100. doi: 10.1016/j.chemphyslip.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Nieva JL, Nir S, Muga A, Goni FM, Wilschut J. Interaction of the HIV-1 fusion peptide with phospholipid vesicles: different structural requirements for fusion and leakage. Biochemistry. 1994;33:3201–9. doi: 10.1021/bi00177a009. [DOI] [PubMed] [Google Scholar]
- 29.Castano S, Desbat B. Structure and orientation study of fusion peptide FP23 of gp41 from HIV-1 alone or inserted into various lipid membrane models (mono-, bi- and multibi-layers) by FT-IR spectroscopies and Brewster angle microscopy. Biochim Biophys Acta. 2005;1715:81–95. doi: 10.1016/j.bbamem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Gordon LM, Nisthal A, Lee AB, Eskandari S, Ruchala P, Jung CL, Waring AJ, Mobley PW. Structural and functional properties of peptides based on the N-terminus of HIV-1 gp41 and the C-terminus of the amyloid-beta protein. Biochim Biophys Acta. 2008;1778:2127–37. doi: 10.1016/j.bbamem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiang W, Weliky DP. HIV fusion peptide and its cross-linked oligomers: efficient syntheses, significance of the trimer in fusion activity, correlation of beta strand conformation with membrane cholesterol, and proximity to lipid headgroups. Biochemistry. 2009;48:289–301. doi: 10.1021/bi8015668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomara MJ, Lorizate M, Huarte N, Mingarro I, Perez-Paya E, Nieva JL. Hexapeptides that interfere with HIV-1 fusion peptide activity in liposomes block GP41-mediated membrane fusion. FEBS Lett. 2006;580:2561–6. doi: 10.1016/j.febslet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Peisajovich SG, Epand RF, Pritsker M, Shai Y, Epand RM. The polar region consecutive to the HIV fusion peptide participates in membrane fusion. Biochemistry. 2000;39:1826–33. doi: 10.1021/bi991887i. [DOI] [PubMed] [Google Scholar]
- 34.Pascual R, Moreno MR, Villalain J. A peptide pertaining to the loop segment of human immunodeficiency virus gp41 binds and interacts with model biomembranes: implications for the fusion mechanism. J Virol. 2005;79:5142–52. doi: 10.1128/JVI.79.8.5142-5152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korazim O, Sackett K, Shai Y. Functional and structural characterization of HIV-1 gp41 ectodomain regions in phospholipid membranes suggests that the fusion-active conformation is extended. J Mol Biol. 2006;364:1103–17. doi: 10.1016/j.jmb.2006.08.091. [DOI] [PubMed] [Google Scholar]
- 36.Verkleij AJ, Zwaal RF, Roelofsen B, Comfurius P, Kastelijn D, van Deenen LL. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973;323:178–93. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- 37.Kiessling V, Wan C, Tamm LK. Domain coupling in asymmetric lipid bilayers. Biochim Biophys Acta. 2009;1788:64–71. doi: 10.1016/j.bbamem.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, van Heerde WL, Henfling ME, Vermeij-Keers C, Schutte B, Borgers M, Ramaekers FC. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631–42. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 39.Gautier I, Coppey J, Durieux C. Early apoptosis-related changes triggered by HSV-1 in individual neuronlike cells. Exp Cell Res. 2003;289:174–83. doi: 10.1016/s0014-4827(03)00258-1. [DOI] [PubMed] [Google Scholar]
- 40.Sackett K, Shai Y. The HIV-1 gp41 N-terminal heptad repeat plays an essential role in membrane fusion. Biochemistry. 2002;41:4678–85. doi: 10.1021/bi0255322. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs A, Sen J, Rong L, Caffrey M. Alanine scanning mutants of the HIV gp41 loop. J Biol Chem. 2005;280:27284–8. doi: 10.1074/jbc.M414411200. [DOI] [PubMed] [Google Scholar]
- 42.Moreno MR, Pascual R, Villalain J. Identification of membrane-active regions of the HIV-1 envelope glycoprotein gp41 using a 15-mer gp41-peptide scan. Biochim Biophys Acta. 2004;1661:97–105. doi: 10.1016/j.bbamem.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Munoz-Barroso I, Salzwedel K, Hunter E, Blumenthal R. Role of the membrane-proximal domain in the initial stages of human immunodeficiency virus type 1 envelope glycoprotein-mediated membrane fusion. J Virol. 1999;73:6089–92. doi: 10.1128/jvi.73.7.6089-6092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vishwanathan SA, Hunter E. Importance of the membrane-perturbing properties of the membrane-proximal external region of human immunodeficiency virus type 1 gp41 to viral fusion. J Virol. 2008;82:5118–26. doi: 10.1128/JVI.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suarez T, Nir S, Goni FM, Saez-Cirion A, Nieva JL. The pre-transmembrane region of the human immunodeficiency virus type-1 glycoprotein: a novel fusogenic sequence. FEBS Lett. 2000;477:145–9. doi: 10.1016/s0014-5793(00)01785-3. [DOI] [PubMed] [Google Scholar]
- 46.Apellaniz B, Nir S, Nieva JL. Distinct mechanisms of lipid bilayer perturbation induced by peptides derived from the membrane-proximal external region of HIV-1 gp41. Biochemistry. 2009;48:5320–31. doi: 10.1021/bi900504t. [DOI] [PubMed] [Google Scholar]
- 47.Barbato G, Bianchi E, Ingallinella P, Hurni WH, Miller MD, Ciliberto G, Cortese R, Bazzo R, Shiver JW, Pessi A. Structural analysis of the epitope of the anti-HIV antibody 2F5 sheds light into its mechanism of neutralization and HIV fusion. J Mol Biol. 2003;330:1101–15. doi: 10.1016/s0022-2836(03)00611-9. [DOI] [PubMed] [Google Scholar]
- 48.Weiss C, White J. Characterization of stable Chinese hamster ovary cells expressing wild-type, secreted, and glycosylphosphatidylinositol-anchored human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1993;67:7060–6. doi: 10.1128/jvi.67.12.7060-7066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salzwedel K, Johnston PB, Roberts SJ, Dubay JW, Hunter E. Expression and characterization of glycophospholipid-anchored human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1993;67:5279–88. doi: 10.1128/jvi.67.9.5279-5288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilk T, Pfeiffer T, Bukovsky A, Moldenhauer G, Bosch V. Glycoprotein incorporation and HIV-1 infectivity despite exchange of the gp160 membrane-spanning domain. Virology. 1996;218:269–74. doi: 10.1006/viro.1996.0190. [DOI] [PubMed] [Google Scholar]
- 51.Miyauchi K, Komano J, Yokomaku Y, Sugiura W, Yamamoto N, Matsuda Z. Role of the specific amino acid sequence of the membrane-spanning domain of human immunodeficiency virus type 1 in membrane fusion. J Virol. 2005;79:4720–9. doi: 10.1128/JVI.79.8.4720-4729.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welman M, Lemay G, Cohen EA. Role of envelope processing and gp41 membrane spanning domain in the formation of human immunodeficiency virus type 1 (HIV-1) fusion-competent envelope glycoprotein complex. Virus Res. 2007;124:103–12. doi: 10.1016/j.virusres.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Shang L, Yue L, Hunter E. Role of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein in cell-cell fusion and virus infection. J Virol. 2008;82:5417–28. doi: 10.1128/JVI.02666-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yue L, Shang L, Hunter E. Truncation of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein defines elements required for fusion, incorporation, and infectivity. J Virol. 2009;83:11588–98. doi: 10.1128/JVI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viard M, Ablan SD, Zhou M, Veenstra TD, Freed EO, Raviv Y, Blumenthal R. Photoinduced reactivity of the HIV-1 envelope glycoprotein with a membrane-embedded probe reveals insertion of portions of the HIV-1 Gp41 cytoplasmic tail into the viral membrane. Biochemistry. 2008;47:1977–83. doi: 10.1021/bi701920f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyss S, Dimitrov A, Baribaud F, Edwards T, Blumenthal R, Hoxie J. Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail. J Virol. 2005;79:12231–41. doi: 10.1128/JVI.79.19.12231-12241.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dimmock NJ. The complex antigenicity of a small external region of the C-terminal tail of the HIV-1 gp41 envelope protein: a lesson in epitope analysis. Rev Med Virol. 2005;15:365–81. doi: 10.1002/rmv.476. [DOI] [PubMed] [Google Scholar]
- 58.Hollier MJ, Dimmock NJ. The C-terminal tail of the gp41 transmembrane envelope glycoprotein of HIV-1 clades A, B, C, and D may exist in two conformations: an analysis of sequence, structure, and function. Virology. 2005;337:284–96. doi: 10.1016/j.virol.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu L, Zhu Y, Huang J, Chen X, Yang H, Jiang S, Chen YH. Surface exposure of the HIV-1 env cytoplasmic tail LLP2 domain during the membrane fusion process: interaction with gp41 fusion core. J Biol Chem. 2008;283:16723–31. doi: 10.1074/jbc.M801083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durell SR, Martin I, Ruysschaert JM, Shai Y, Blumenthal R. What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion (review) Mol Membr Biol. 1997;14:97–112. doi: 10.3109/09687689709048170. [DOI] [PubMed] [Google Scholar]
- 61.Stegmann T, Nir S, Wilschut J. Membrane fusion activity of influenza virus. Effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry. 1989;28:1698–704. doi: 10.1021/bi00430a041. [DOI] [PubMed] [Google Scholar]
- 62.Dimitrov AS, Xiao X, Dimitrov DS, Blumenthal R. Early intermediates in HIV-1 envelope glycoprotein-mediated fusion triggered by CD4 and co-receptor complexes. J Biol Chem. 2001;276:30335–41. doi: 10.1074/jbc.M103788200. [DOI] [PubMed] [Google Scholar]
- 63.Dimitrov AS, Louis JM, Bewley CA, Clore GM, Blumenthal R. Conformational changes in HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion and inactivation. Biochemistry. 2005;44:12471–9. doi: 10.1021/bi051092d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dimitrov AS, Jacobs A, Finnegan CM, Stiegler G, Katinger H, Blumenthal R. Exposure of the membrane-proximal external region of HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion. Biochemistry. 2007;46:1398–401. doi: 10.1021/bi062245f. [DOI] [PubMed] [Google Scholar]
- 65.Melikyan G, Markosyan R, Hemmati H, Delmedico M, Lambert D, Cohen F. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol. 2000;151:413–23. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mobley PW, Barry JA, Waring AJ, Sherman MA, Gordon LM. Membrane perturbing actions of HIV type 1 glycoprotein 41 domains are inhibited by helical C-peptides. AIDS Res Hum Retroviruses. 2007;23:224–42. doi: 10.1089/aid.2006.0046. [DOI] [PubMed] [Google Scholar]
- 67.Owens RJ, Tanner CC, Mulligan MJ, Srinivas RV, Compans RW. Oligopeptide inhibitors of HIV-induced syncytium formation. AIDS Res Hum Retroviruses. 1990;6:1289–96. doi: 10.1089/aid.1990.6.1289. [DOI] [PubMed] [Google Scholar]
- 68.Pritsker M, Jones P, Blumenthal R, Shai Y. A synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 recognizes the wild-type fusion peptide in the membrane and inhibits HIV-1 envelope glycoprotein-mediated cell fusion. Proc Natl Acad Sci U S A. 1998;95:7287–92. doi: 10.1073/pnas.95.13.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wild C, Shugars D, Greenwell T, McDanal C, Matthews T. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A. 1994;91:9770–4. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang S, Lin K, Strick N, Neurath A. HIV-1 inhibition by a peptide. Nature. 1993;365:113. doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 71.Yu H, Tudor D, Alfsen A, Labrosse B, Clavel F, Bomsel M. Peptide P5 (residues 628–683), comprising the entire membrane proximal region of HIV-1 gp41 and its calcium-binding site, is a potent inhibitor of HIV-1 infection. Retrovirology. 2008;5:93. doi: 10.1186/1742-4690-5-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Forsell MN, Schief WR, Wyatt RT. Immunogenicity of HIV-1 envelope glycoprotein oligomers. Curr Opin HIV AIDS. 2009;4:380–7. doi: 10.1097/COH.0b013e32832edc19. [DOI] [PubMed] [Google Scholar]
- 74.Mascola JR, Montefiori DC. The Role of Antibodies in HIV Vaccines. Annu Rev Immunol. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 75.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78:10724–37. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–8. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 77.Matyas GR, Beck Z, Karasavvas N, Alving CR. Lipid binding properties of 4E10, 2F5, and WR304 monoclonal antibodies that neutralize HIV-1. Biochim Biophys Acta. 2009;1788:660–5. doi: 10.1016/j.bbamem.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez-Martinez S, Lorizate M, Hermann K, Kunert R, Basanez G, Nieva JL. Specific phospholipid recognition by human immunodeficiency virus type-1 neutralizing anti-gp41 2F5 antibody. FEBS Lett. 2006;580:2395–99. doi: 10.1016/j.febslet.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 79.Liu S, Wu S, Jiang S. HIV entry inhibitors targetinggp41: from polypeptides to small-molecule compounds. Curr Pharm Des. 2007;13:143–62. doi: 10.2174/138161207779313722. [DOI] [PubMed] [Google Scholar]
- 80.Liu S, Lu H, Niu J, Xu Y, Wu S, Jiang S. Different from the HIV fusion inhibitor C34, the anti-HIV drug Fuzeon (T-20) inhibits HIV-1 entry by targeting multiple sites in gp41 and gp120. J Biol Chem. 2005;280:11259–73. doi: 10.1074/jbc.M411141200. [DOI] [PubMed] [Google Scholar]
- 81.Aquaro S, D’Arrigo R, Svicher V, Perri G, Caputo S, Visco-Comandini U, Santoro M, Bertoli A, Mazzotta F, Bonora S, Tozzi V, Bellagamba R, Zaccarelli M, Narciso P, Antinori A, Perno C. Specific mutations in HIV-1 gp41 are associated with immunological success in HIV-1-infected patients receiving enfuvirtide treatment. J Antimicrob Chemother. 2006;58:714–22. doi: 10.1093/jac/dkl306. [DOI] [PubMed] [Google Scholar]
- 82.Svicher V, Aquaro S, D’Arrigo R, Artese A, Dimonte S, Alcaro S, Santoro M, Di Perri G, Caputo S, Bellagamba R, Zaccarelli M, Visco-Comandini U, Antinori A, Narciso P, Ceccherini-Silberstein F, Perno C. Specific enfuvirtide-associated mutational pathways in HIV-1 Gp41 are significantly correlated with an increase in CD4(+) cell count, despite virological failure. J Infect Dis. 2008;197:1408–18. doi: 10.1086/587693. [DOI] [PubMed] [Google Scholar]
- 83.Melby T, Despirito M, Demasi R, Heilek G, Thommes J, Greenberg M, Graham N. Association between specific enfuvirtide resistance mutations and CD4 cell response during enfuvirtide-based therapy. AIDS. 2007;21:2537–9. doi: 10.1097/QAD.0b013e3282f12362. [DOI] [PubMed] [Google Scholar]
- 84.Sista PR, Melby T, Davison D, Jin L, Mosier S, Mink M, Nelson EL, DeMasi R, Cammack N, Salgo MP, Matthews TJ, Greenberg ML. Characterization of determinants of genotypic and phenotypic resistance to enfuvirtide in baseline and on-treatment HIV-1 isolates. AIDS. 2004;18:1787–94. doi: 10.1097/00002030-200409030-00007. [DOI] [PubMed] [Google Scholar]
- 85.Chinnadurai R, Rajan D, Munch J, Kirchhoff F. Human immunodeficiency virus type 1 variants resistant to first- and second-version fusion inhibitors and cytopathic in ex vivo human lymphoid tissue. J Virol. 2007;81:6563–72. doi: 10.1128/JVI.02546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lalezari JP, Bellos NC, Sathasivam K, Richmond GJ, Cohen CJ, Myers RA, Jr, Henry DH, Raskino C, Melby T, Murchison H, Zhang Y, Spence R, Greenberg ML, Demasi RA, Miralles GD. T-1249 retains potent antiretroviral activity in patients who had experienced virological failure while on an enfuvirtide-containing treatment regimen. J Infect Dis. 2005;191:1155–63. doi: 10.1086/427993. [DOI] [PubMed] [Google Scholar]
- 87.Dwyer JJ, Wilson KL, Davison DK, Freel SA, Seedorff JE, Wring SA, Tvermoes NA, Matthews TJ, Greenberg ML, Delmedico MK. Design of helical, oligomeric HIV-1 fusion inhibitor peptides with potent activity against enfuvirtide-resistant virus. Proc Natl Acad Sci U S A. 2007;104:12772–7. doi: 10.1073/pnas.0701478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He Y, Xiao Y, Song H, Liang Q, Ju D, Chen X, Lu H, Jing W, Jiang S, Zhang L. Design and evaluation of sifuvirtide, a novel HIV-1 fusion inhibitor. J Biol Chem. 2008;283:11126–34. doi: 10.1074/jbc.M800200200. [DOI] [PubMed] [Google Scholar]
- 89.Wang RR, Yang LM, Wang YH, Pang W, Tam SC, Tien P, Zheng YT. Sifuvirtide, a potent HIV fusion inhibitor peptide. Biochem Biophys Res Commun. 2009;382:540–4. doi: 10.1016/j.bbrc.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 90.Jacobs A, Quraishi O, Huang X, Bousquet-Gagnon N, Nault G, Francella N, Alvord WG, Pham N, Soucy C, Robitaille M, Bridon D, Blumenthal R. A covalent inhibitor targeting an intermediate conformation of the fusogenic subunit of the HIV-1 envelope complex. J Biol Chem. 2007;282:32406–13. doi: 10.1074/jbc.M705577200. [DOI] [PubMed] [Google Scholar]
- 91.Ingallinella P, Bianchi E, Ladwa NA, Wang YJ, Hrin R, Veneziano M, Bonelli F, Ketas TJ, Moore JP, Miller MD, Pessi A. Addition of a cholesterol group to an HIV-1 peptide fusion inhibitor dramatically increases its antiviral potency. Proc Natl Acad Sci U S A. 2009;106:5801–6. doi: 10.1073/pnas.0901007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stoddart CA, Nault G, Galkina SA, Thibaudeau K, Bakis P, Bousquet-Gagnon N, Robitaille M, Bellomo M, Paradis V, Liscourt P, Lobach A, Rivard ME, Ptak RG, Mankowski MK, Bridon D, Quraishi O. Albumin-conjugated C34 peptide HIV-1 fusion inhibitor: equipotent to C34 and T-20 in vitro with sustained activity in SCID-hu Thy/Liv mice. J Biol Chem. 2008;283:34045–52. doi: 10.1074/jbc.M805536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pan C, Liu S, Jiang S. HIV-1 gp41 Fusion Intermediate: A Target for HIV Therapeutics. J Formos Med Assoc. 109:94–105. doi: 10.1016/S0929-6646(10)60029-0. [DOI] [PubMed] [Google Scholar]
- 94.Eckert DM, Malashkevich VN, Hong LH, Carr PA, Kim PS. Inhibiting HIV-1 entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell. 1999;99:103–15. doi: 10.1016/s0092-8674(00)80066-5. [DOI] [PubMed] [Google Scholar]
- 95.Welch BD, VanDemark AP, Heroux A, Hill CP, Kay MS. Potent D-peptide inhibitors of HIV-1 entry. Proc Natl Acad Sci U S A. 2007;104:16828–33. doi: 10.1073/pnas.0708109104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welch BD, Francis JN, Redman JS, Paul S, Weinstock MT, Reeves JD, Lie YS, Whitby FG, Eckert DM, Hill CP, Root MJ, Kay MS. Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance. J Virol. 2010;84:11235–44. doi: 10.1128/JVI.01339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wexler-Cohen Y, Ashkenazi A, Viard M, Blumenthal R, Shai Y. Virus-cell and cell-cell fusion mediated by the HIV-1 envelope glycoprotein is inhibited by short gp41 N-terminal membrane-anchored peptides lacking the critical pocket domain. FASEB J. 2010;24:4196–202. doi: 10.1096/fj.09-151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deng Y, Zheng Q, Ketas TJ, Moore JP, Lu M. Protein design of a bacterially expressed HIV-1 gp41 fusion inhibitor. Biochemistry. 2007;46:4360–9. doi: 10.1021/bi7001289. [DOI] [PubMed] [Google Scholar]
- 99.Ketas TJ, Schader SM, Zurita J, Teo E, Polonis V, Lu M, Klasse PJ, Moore JP. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology. 2007;364:431–40. doi: 10.1016/j.virol.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Chen X, Lu L, Qi Z, Lu H, Wang J, Yu X, Chen Y, Jiang S. Novel recombinant engineered gp41 N-terminal heptad repeat trimers and their potential as anti-HIV-1 therapeutics or microbicides. J Biol Chem. 2010;285:25506–15. doi: 10.1074/jbc.M110.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan DC, Chutkowski CT, Kim PS. Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target. Proc Natl Acad Sci U S A. 1998;95:15613–7. doi: 10.1073/pnas.95.26.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu S, Jiang S. High throughput screening and characterization of HIV-1 entry inhibitors targeting gp41: theories and techniques. Curr Pharm Des. 2004;10:1827–43. doi: 10.2174/1381612043384466. [DOI] [PubMed] [Google Scholar]
- 103.Zhao Q, Ernst JT, Hamilton AD, Debnath AK, Jiang S. XTT formazan widely used to detect cell viability inhibits HIV type 1 infection in vitro by targeting gp41. AIDS Res Hum Retroviruses. 2002;18:989–97. doi: 10.1089/08892220260235353. [DOI] [PubMed] [Google Scholar]
- 104.Wang H, Qi Z, Guo A, Mao Q, Lu H, An X, Xia C, Li X, Debnath AK, Wu S, Liu S, Jiang S. ADS-J1 inhibits human immunodeficiency virus type 1 entry by interacting with the gp41 pocket region and blocking fusion-active gp41 core formation. Antimicrob Agents Chemother. 2009;53:4987–98. doi: 10.1128/AAC.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stewart KD, Huth JR, Ng TI, McDaniel K, Hutchinson RN, Stoll VS, Mendoza RR, Matayoshi ED, Carrick R, Mo H, Severin J, Walter K, Richardson PL, Barrett LW, Meadows R, Anderson S, Kohlbrenner W, Maring C, Kempf DJ, Molla A, Olejniczak ET. Non-peptide entry inhibitors of HIV-1 that target the gp41 coiled coil pocket. Bioorg Med Chem Lett. 20:612–7. doi: 10.1016/j.bmcl.2009.11.076. [DOI] [PubMed] [Google Scholar]
- 106.Katritzky AR, Tala SR, Lu H, Vakulenko AV, Chen QY, Sivapackiam J, Pandya K, Jiang S, Debnath AK. Design, synthesis, and structure-activity relationship of a novel series of 2-aryl 5-(4-oxo-3-phenethyl-2-thioxothiazolidinylidenemethyl)furans as HIV-1 entry inhibitors. J Med Chem. 2009;52:7631–9. doi: 10.1021/jm900450n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu K, Lu H, Hou L, Qi Z, Teixeira C, Barbault F, Fan BT, Liu S, Jiang S, Xie L. Design, synthesis, and biological evaluation of N-carboxyphenylpyrrole derivatives as potent HIV fusion inhibitors targeting gp41. J Med Chem. 2008;51:7843–54. doi: 10.1021/jm800869t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frey G, Rits-Volloch S, Zhang XQ, Schooley RT, Chen B, Harrison SC. Small molecules that bind the inner core of gp41 and inhibit HIV envelope-mediated fusion. Proc Natl Acad Sci U S A. 2006;103:13938–43. doi: 10.1073/pnas.0601036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cai L, Balogh E, Gochin M. Stable extended human immunodeficiency virus type 1 gp41 coiled coil as an effective target in an assay for high-affinity fusion inhibitors. Antimicrob Agents Chemother. 2009;53:2444–9. doi: 10.1128/AAC.00150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou G, Wu D, Hermel E, Balogh E, Gochin M. Design, synthesis, and evaluation of indole compounds as novel inhibitors targeting Gp41. Bioorg Med Chem Lett. 20:1500–3. doi: 10.1016/j.bmcl.2010.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Silvestri G, Sodora D, Koup R, Paiardini M, O’Neil S, McClure H, Staprans S, Feinberg M. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–52. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 112.Pandrea I, Sodora D, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–28. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dunham R, Pagliardini P, Gordon S, Sumpter B, Engram J, Moanna A, Paiardini M, Mandl J, Lawson B, Garg S, McClure H, Xu Y, Ibegbu C, Easley K, Katz N, Pandrea I, Apetrei C, Sodora D, Staprans S, Feinberg M, Silvestri G. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood. 2006;108:209–17. doi: 10.1182/blood-2005-12-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gordon S, Klatt N, Bosinger S, Brenchley J, Milush J, Engram J, Dunham R, Paiardini M, Klucking S, Danesh A, Strobert E, Apetrei C, Pandrea I, Kelvin D, Douek D, Staprans S, Sodora D, Silvestri G. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–34. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Finkel T, Tudor-Williams G, Banda N, Cotton M, Curiel T, Monks C, Baba T, Ruprecht R, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV-and SIV-infected lymph nodes. Nat Med. 1995;1:129–34. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 116.Biard-Piechaczyk M, Robert-Hebmann V, Roland J, Coudronniere N, Devaux C. Role of CXCR4 in HIV-1-induced apoptosis of cells with a CD4+, CXCR4+ phenotype. Immunol Lett. 1999;70:1–3. doi: 10.1016/s0165-2478(99)00124-8. [DOI] [PubMed] [Google Scholar]
- 117.Jacotot E, Krust B, Callebaut C, Laurent-Crawford A, Blanco J, Hovanessian A. HIV-1 envelope glycoproteins-mediated apoptosis is regulated by CD4 dependent and independent mechanisms. Apoptosis. 1997;2:47–60. doi: 10.1023/a:1026435625144. [DOI] [PubMed] [Google Scholar]
- 118.Laurent-Crawford A, Krust B, Riviere Y, Desgranges C, Muller S, Kieny M, Dauguet C, Hovanessian A. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–73. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 119.Blanco J, Barretina J, Henson G, Bridger G, De Clercq E, Clotet B, Este J. The CXCR4 antagonist AMD3100 efficiently inhibits cell-surface-expressed human immunodeficiency virus type 1 envelope-induced apoptosis. Antimicrob Agents Chemother. 2000;44:51–6. doi: 10.1128/aac.44.1.51-56.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holm G, Zhang C, Gorry P, Peden K, Schols D, De Clercq E, Gabuzda D. Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J Virol. 2004;78:4541–51. doi: 10.1128/JVI.78.9.4541-4551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind R, Devaux C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268:329–44. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- 122.Roggero R, Robert-Hebmann V, Harrington S, Roland J, Vergne L, Jaleco S, Devaux C, Biard-Piechaczyk M. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J Virol. 2001;75:7637–50. doi: 10.1128/JVI.75.16.7637-7650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Blanco J, Jacotot E, Cabrera C, Cardona A, Clotet B, De Clercq E, Este J. The implication of the chemokine receptor CXCR4 in HIV-1 envelope protein-induced apoptosis is independent of the G protein-mediated signalling. AIDS. 1999;13:909–17. doi: 10.1097/00002030-199905280-00006. [DOI] [PubMed] [Google Scholar]
- 124.Scheller C, Jassoy C. Syncytium formation amplifies apoptotic signals: a new view on apoptosis in HIV infection in vitro. Virology. 2001;282:48–55. doi: 10.1006/viro.2000.0811. [DOI] [PubMed] [Google Scholar]
- 125.Blanco J, Barretina J, Ferri K, Jacotot E, Gutierrez A, Armand-Ugon M, Cabrera C, Kroemer G, Clotet B, Este J. Cell-surface-expressed HIV-1 envelope induces the death of CD4 T cells during GP41-mediated hemifusion-like events. Virology. 2003;305:318–29. doi: 10.1006/viro.2002.1764. [DOI] [PubMed] [Google Scholar]
- 126.Schuitemaker H, Koot M, Kootstra N, Dercksen M, de Goede R, van Steenwijk R, Lange J, Schattenkerk J, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–60. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koot M, van’t Wout A, Kootstra N, de Goede R, Tersmette M, Schuitemaker H. Relation between changes in cellular load, evolution of viral phenotype, and the clonal composition of virus populations in the course of human immunodeficiency virus type 1 infection. J Infect Dis. 1996;173:349–54. doi: 10.1093/infdis/173.2.349. [DOI] [PubMed] [Google Scholar]
- 128.Spijkerman I, de Wolf F, Langendam M, Schuitemaker H, Coutinho R. Emergence of syncytium-inducing human immunodeficiency virus type 1 variants coincides with a transient increase in viralRNA level and is an independent predictor for progression to AIDS. J Infect Dis. 1998;178:397–403. doi: 10.1086/515627. [DOI] [PubMed] [Google Scholar]
- 129.van Rij RP, Blaak H, Visser J, Brouwer M, Rientsma R, Broersen S, de Roda Husman AM, Schuitemaker H. Differential coreceptor expression allows for independent evolution of non-syncytium-inducing and syncytium-inducing HIV-1. J Clin Invest. 2000;106:1569. doi: 10.1172/jci7953c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karlsson I, Grivel J, Chen S, Karlsson A, Albert J, Fenyo E, Margolis L. Differential pathogenesis of primary CCR5-using human immunodeficiency virus type 1 isolates in ex vivo human lymphoid tissue. J Virol. 2005;79:11151–60. doi: 10.1128/JVI.79.17.11151-11160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sterjovski J, Churchill M, Ellett A, Gray L, Roche M, Dunfee R, Purcell D, Saksena N, Wang B, Sonza S, Wesselingh S, Karlsson I, Fenyo E, Gabuzda D, Cunningham A, Gorry P. Asn 362 in gp120 contributes to enhanced fusogenicity by CCR5-restricted HIV-1 envelope glycoprotein variants from patients with AIDS. Retrovirology. 2007;4:89. doi: 10.1186/1742-4690-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wade J, Sterjovski J, Gray L, Roche M, Chiavaroli L, Ellett A, Jakobsen MR, Cowley D, Pereira Cda F, Saksena N, Wang B, Purcell DF, Karlsson I, Fenyo EM, Churchill M, Gorry PR. Enhanced CD4+ cellular apoptosis by CCR5-restricted HIV-1 envelope glycoprotein variants from patients with progressive HIV-1 infection. Virology. 396:246–55. doi: 10.1016/j.virol.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 133.Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, Sodroski J, Letvin NL. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–8. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karlsson G, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N, Marcon L, Margolin D, Fanton J, Axthelm M, Letvin N, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–71. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Etemad-Moghadam B, Sun Y, Nicholson E, Fernandes M, Liou K, Gomila R, Lee J, Sodroski J. Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo. J Virol. 2000;74:4433–40. doi: 10.1128/jvi.74.9.4433-4440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.LaBonte J, Patel T, Hofmann W, Sodroski J. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J Virol. 2000;74:10690–8. doi: 10.1128/jvi.74.22.10690-10698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Etemad-Moghadam B, Rhone D, Steenbeke T, Sun Y, Manola J, Gelman R, Fanton J, Racz P, Tenner-Racz K, Axthelm M, Letvin N, Sodroski J. Membrane-fusing capacity of the human immunodeficiency virus envelope proteins determines the efficiency of CD+ T-cell depletion in macaques infected by a simian-human immunodeficiency virus. J Virol. 2001;75:5646–55. doi: 10.1128/JVI.75.12.5646-5655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ferri K, Jacotot E, Leduc P, Geuskens M, Ingber D, Kroemer G. Apoptosis of syncytia induced by the HIV-1-envelope glycoprotein complex: influence of cell shape and size. Exp Cell Res. 2000;261:119–26. doi: 10.1006/excr.2000.5062. [DOI] [PubMed] [Google Scholar]
- 139.Ferri K, Jacotot E, Geuskens M, Kroemer G. Apoptosis and karyogamy in syncytia induced by the HIV-1-envelope glycoprotein complex. Cell Death Differ. 2000;7:1137–9. doi: 10.1038/sj.cdd.4400748. [DOI] [PubMed] [Google Scholar]
- 140.Ferri K, Jacotot E, Blanco J, Este J, Zamzami N, Susin S, Xie Z, Brothers G, Reed J, Penninger J, Kroemer G. Apoptosis control in syncytia induced by the HIV type 1-envelope glycoprotein complex: role of mitochondria and caspases. J Exp Med. 2000;192:1081–92. doi: 10.1084/jem.192.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Perfettini J, Roumier T, Castedo M, Larochette N, Boya P, Raynal B, Lazar V, Ciccosanti F, Nardacci R, Penninger J, Piacentini M, Kroemer G. NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J Exp Med. 2004;199:629–40. doi: 10.1084/jem.20031216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perfettini J, Castedo M, Nardacci R, Ciccosanti F, Boya P, Roumier T, Larochette N, Piacentini M, Kroemer G. Essential role of p53 phosphorylation by p38 MAPK in apoptosis induction by the HIV-1 envelope. J Exp Med. 2005;201:279–89. doi: 10.1084/jem.20041502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Perfettini J, Nardacci R, Seror C, Bourouba M, Subra F, Gros L, Manic G, Amendola A, Masdehors P, Rosselli F, Ojcius D, Auclair C, de The H, Gougeon M, Piacentini M, Kroemer G. The tumor suppressor protein PML controls apoptosis induced by the HIV-1 envelope. Cell Death Differ. 2009;16:298–311. doi: 10.1038/cdd.2008.158. [DOI] [PubMed] [Google Scholar]
- 144.Perfettini J, Nardacci R, Bourouba M, Subra F, Gros L, Seror C, Manic G, Rosselli F, Amendola A, Masdehors P, Chessa L, Novelli G, Ojcius D, Siwicki J, Chechlinska M, Auclair C, Regueiro J, de The H, Gougeon M, Piacentini M, Kroemer G. Critical involvement of the ATM-dependent DNA damage response in the apoptotic demise of HIV-1-elicited syncytia. PLoS ONE. 2008;3:e2458. doi: 10.1371/journal.pone.0002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meissner E, Zhang L, Jiang S, Su L. Fusion-induced apoptosis contributes to thymocyte depletion by a pathogenic human immunodeficiency virus type 1 envelope in the human thymus. J Virol. 2006;80:11019–30. doi: 10.1128/JVI.01382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kemble G, Danieli T, White J. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–91. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 147.Chernomordik L, Kozlov M. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123:375–82. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 148.Bar S, Alizon M. Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process. J Virol. 2004;78:811–20. doi: 10.1128/JVI.78.2.811-820.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garg H, Joshi A, Freed E, Blumenthal R. Site-specific mutations in HIV-1 gp41 reveal a correlation between HIV-1-mediated bystander apoptosis and fusion/hemifusion. J Biol Chem. 2007;282:16899–906. doi: 10.1074/jbc.M701701200. [DOI] [PubMed] [Google Scholar]
- 150.Garg H, Blumenthal R. HIV gp41-induced apoptosis is mediated by caspase-3-dependent mitochondrial depolarization, which is inhibited by HIV protease inhibitor nelfinavir. J Leukoc Biol. 2006;79:351–62. doi: 10.1189/jlb.0805430. [DOI] [PubMed] [Google Scholar]
- 151.Garg H, Joshi A, Blumenthal R. Altered bystander apoptosis induction and pathogenesis of enfuvirtide-resistant HIV type 1 Env mutants. AIDS Res Hum Retroviruses. 2009;25:811–7. doi: 10.1089/aid.2009.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garg H, Joshi A, Ye C, Shankar P, Manjunath N. Single amino acid change in gp41 region of HIV-1 alters bystander apoptosis and CD4 decline in humanized mice. Virol J. 8:34. doi: 10.1186/1743-422X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jolly C, Kashefi K, Hollinshead M, Sattentau Q. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–93. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jolly C, Mitar I, Sattentau Q. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol. 2007;81:13916–21. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114:605–10. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]