Abstract
Induction of DNA damage by oxidants such as H2O2 activates the complex network of DNA damage response (DDR) pathways present in cells to initiate DNA repair, halt cell cycle progression, and prepare an apoptotic reaction. We have previously reported that activation of Ataxia Telangiectasia Mutated protein kinase (ATM) and induction of γH2AX are among the early events of the DDR induced by exposure of cells to H2O2, and in human pulmonary carcinoma A549 cells, both events were expressed predominantly during S-phase. This study was designed to further explore a correlation between these events and DNA replication. Toward this end, we utilized 5-ethynyl-2′deoxyuridine (EdU) and the “click chemistry” approach to label DNA during replication, followed by exposure of A549 cells to H2O2. Multiparameter laser scanning cytometric analysis of these cells made it possible to identify DNA replicating cells and directly correlate H2O2-induced ATM activation and induction of γH2AX with DNA replication on a cell by cell basis. After pulse-labeling with EdU and exposure to H2O2, confocal microscopy was also used to examine the localization of DNA replication sites (“replication factories”) versus the H2AX phosphorylation sites (γH2AX foci) in nuclear chromatin in an attempt to observe the absence or presence of colocalization. The data indicate a close association between DNA replication and H2AX phosphorylation in A549 cells, suggesting that these DNA damage response events may be triggered by stalled replication forks and perhaps also by induction of DNA double-strand breaks at the primary DNA lesions induced by H2O2
Key terms: reactive oxygen species, hydrogen peroxide, S phase, cell cycle, EdU incorporation, DNA damage response, replication stress
DNA in live cells is being persistently damaged by reactive oxygen species that are either the by-products of aerobic respiration (1–9), generated during macrophage oxidative burst (10–12), originating from environmental pollutants (13) or even of iatrogenic origin (14). The damage manifests as oxidation of DNA bases with a prevalence of guanine adducts such as 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG)], fragmentation of base rings, sugar modification, DNA and protein crosslinking, and induction of DNA strand breaks (DSBs) (2,15,16). Repair of DSBs can be error prone leading to deletion of base pairs and producing defects that result in translocations and chromosomal instability (3,4,17,18). Alterations in molecular structure of DNA accumulating with time are important factors in promoting aging, cellular senescence, and predisposing to cancer (1–6,19–21).
In our prior publications, we addressed the issue of oxidative DNA damage such as that induced by endogenous oxidants produced during aerobic metabolism (8,9,22–24), generated by the oxidative burst of macrophages (25) or by cell treatment with nitrogen oxide-releasing aspirin (26). In these studies, DNA damage was monitored indirectly, by assessment of histone H2AX phosphorylation (expression of γH2AX) (27) and activation of Ataxia Telangiectasia Mutated protein kinase (ATM) through its autophosphorylation on Ser1981. Phospho-specific Abs and multiparameter cytometry were used to detect these phosphorylation events and correlate them with the cell cycle phase and induction of apoptosis. Our studies provided evidence that DNA damage signaling induced by the oxidants revealed by H2AX phosphorylation, and ATM activation was strongly correlated with progression of cells through the S phase of the cell cycle (8,9,22–26).
This study was designed to obtain direct evidence on the possible association between DNA replication and H2AX phosphorylation as well as ATM activation upon induction of oxidative DNA damage. Toward this end, we have used the “click chemistry” approach to label DNA being actually replicated with 5-ethynyl-2′deoxyuridine (EdU) (28–30) followed by cells exposure to the oxidizing/DNA damaging agent H2O2. Analysis of these cells by multiparameter cytometry made it possible to identify DNA replicating cells and directly correlate the H2O2-induced ATM activation and expression of γH2AX with DNA replication on a cell by cell basis (29). Visualization of nuclear sites of DNA replication (“replication factories”) through their nascent labeling with EdU versus the sites of H2AX phosphorylation (γH2AX foci) was accomplished by confocal microscopy. This approach made it possible to observe whether these events were spatially colocalized.
Materials and Methods
Cells, Cell Treatment
Human lung carcinoma A549 cells were purchased from American Type Culture Collection (ATCC CCL-185, Manassas, VA). The cells were cultured in Ham’s F12K medium with 2 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate (ATCC) and supplemented with 10% fetal bovine serum (ATCC). Dual-chambered slides (Nunc Lab-Tek II) were seeded with 105 cells/ml suspended in 1 ml medium per chamber. During treatment with EdU and H2O2, the cells were in exponential phase of growth. The cultures were treated with 10 μM EdU (Invitrogen/Molecular Probes, Eugene, OR) for 60 min and then, while still in the presence of EdU, with 200 μM H2O2 (Sigma Chemical Co., St Louis, MO) for another 60 min. The cells were then rinsed with phosphate buffered salt solution (PBS) and fixed by transferring slides into Coplin jars containing 1% methanol-free formaldehyde (Polysciences, Warrington, PA) for 15 min. The slides were postfixed in 70% ethanol and kept at 0–4°C until staining.
Detection of H2AX Phosphorylation, ATM Activation, and Incorporation of EdU
Slides in fixative were washed twice in PBS and the cells on the slides treated with 0.1% Triton X-100 (Sigma) in PBS for 15 min, and with a 1% (w/v) solution of bovine serum albumin (BSA; Sigma) in PBS for 30 min (or overnight incubation for confocal imaging) to suppress nonspecific antibody (Ab) binding. The cells were then incubated in a 100 μl volume of 1% BSA containing a 1:300 dilution of phospho-specific (Ser139) γH2AX mAb (Biolegend, San Diego, CA or with 1:100 dilution of phospho-specific (Ser1981) ATM mAb (Millipore, Tamecula, CA). The secondary Ab was tagged with AlexaFluor 647 fluorochrome (Invitrogen/Molecular Probes, used at 1:200 dilution). The Click-iT® EdU AlexaFluor® 488 imaging kit (Invitrogen/Molecular Probes) was used to detect EdU incorporation. Before measurement by LSC, the cells were counterstained with 2.8 μg/ml 4,6-diamidino-2-pheny-lindole (DAPI; Sigma) in PBS for 15 min. Each experiment was performed with an IgG control in which cells were labeled only with the secondary AlexaFluor 647 antibody, without primary antibody incubation to estimate the extent of nonspecific binding of the secondary antibody to the cells. Other details of cell incubations with the primary and secondary Ab have been previously described (31,32).
Measurement of Cell Fluorescence by LSC
Cellular green (EdU) or red IF representing the binding of the respective phospho-specific Abs as well as the blue emission of DAPI stained DNA was measured using an LSC (iCys; CompuCyte, Westwood, MA) utilizing standard filter settings; fluorescence was excited with 488-nm argon, helium-neon (633 nm) and violet (405 nm) lasers (33). The intensities of maximal pixel and integrated fluorescence were measured and recorded for each cell. At least 3,000 cells were measured per sample. Gating analysis was carried out as described in Figure legends. To measure the correlation between incorporation of EdU and expression of γH2AX or ATM-S1981P, the subpopulations of DNA replicating cells were gated as shown in Figures 1–3, and the Microsoft Excel was used to analyze the correlation coefficient (Pearson’s r) within these subpopulations.
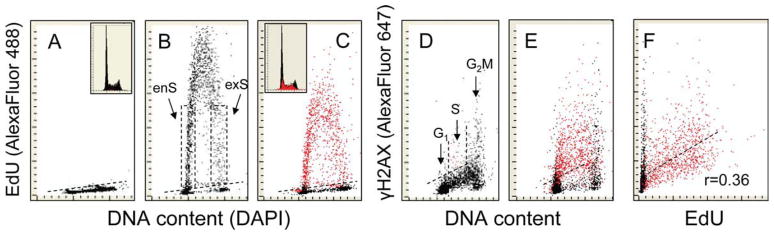
Figure 1.
Correlation between DNA replication and induction of γH2AX in A549 cells treated with H2O2. Panel A: The cells were not exposed to EdU nor were they treated with H2O2. Panels B and D: cells were exposed in culture to 5 μM EdU for 120 min. The cells with variable level of EdU incorporation were entering (enS) or exiting (exS) S phase during the duration of the EdU pulse as their interval of exposure to the precursor at the time of DNA replication varied between 1 and 120 min. C, E, and F: The cultures were initially treated with 5 μM EdU for 60 min, then exposed to 200 μM of H2O2 (still in the presence of EdU) for an additional 60 min. All cultures (B—F) thus had a 120 min-pulse of EdU, while C,E,F cultures were also exposed to H2O2 for 60 min. Using the “paint-a-gate” gating analysis, the DNA replicating cells characterized by EdU incorporation (above the threshold of the of unexposed to EdU cells; A, dashed line) were colored red (B) and then replotted as γH2AX vs. DNA content (E) or γH2AX vs. EdU (F) bivariate distributions. It is quite evident that following exposure to H2O2, predominantly DNA replicating (red) cells had increased expression of γH2AX [above the level of the H2O2 untreated control cells (D, dashed line)]. A moderate correlation between EdU incorporation and the induction of H2AX estimated for the population of DNA replicating (red colored) cells is apparent, with the coefficient (Pearson) r = 0.36 (F). The insets in A and C show DNA frequency histograms from the respective cultures. [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
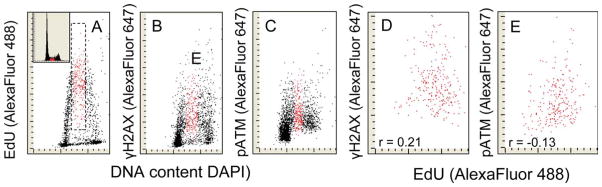
Figure 3.
Relationship between the induction of γH2AX or activation of ATM and the extent of EdU incorporation of cells exposed to H2O2. Panel A: The cells were exposed to EdU for 60 min and then to H2O2 for the next 60 min (as in Fig. 1C). The analysis gate was set in mid-S to exclude the enS and exS cells. Such analysis enabled one to correlate the extent of EdU incorporation of the cells that were exposed to EdU for the full 120 min at the time when they were replicating DNA compared to the expression of γH2AX (D) or ATM-S1981P(E). [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
Mapping Replication and H2AX Phosphorylation by Confocal Microscopy
Cell cultures intended for imaging experiments were grown on round coverslips submerged in Petri dishes. Cells were incubated with EdU first (30 min; Click-iT EdU Alexa-Fluor 488 Imaging Kit cat. # C10337; Invitrogen/Molecular Probes) and H2O2 was added at final concentration 100 μM for additional 30 min in the presence of EdU. Cells were fixed with methanol-free formaldehyde (4%) and treated with Triton X-100 (0.1%). Blocking was done overnight in 3% (w/v) BSA; phospho-specific (Ser139) γH2AX mAb (Upstate Bio-technology Inc., Lake Placid, NY) was used, followed by a secondary antibody AlexaFluor 568 goat anti-mouse IgG (H+L), cat. # A11004 (Invitrogen/Molecular Probes). Subsequently, detection of EdU was performed, according to manufacturer’s instructions. Images were recorded using Leica LSC SP5 confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany). The following instrumental parameters were used: 63× HCX PL APO CS NA 1.4 oil immersion lens, excitation 488 (Ar) and 561 nm (HeNe), emission detection bands 500–550 nm for AlexaFluor488 (Click-iT EdU) and 600 to 660 nm for AlexaFluor568 (immunofluorescence, γH2A.X), registration in sequential mode, scanning or 8000 Hz (resonant scanner), with 16–32 averaged frames. Images were deconvoluted using Auto-Deblur software v.X2.2 (Media Cybernetics, USA).
Results
As mentioned, the detection of EdU incorporation using the “click chemistry” approach enables one to identify DNA replicating cells and concurrently detect other intracellular epitopes immunocytochemically (28–30). This approach has been presently used to correlate DNA replication and H2AX or ATM phosphorylation in cells subjected to oxidative stress. To label nascent DNA, the cells were treated with EdU for 60 min and then, still in the presence of EdU, exposed to H2O2 for an additional hour to induce γH2AX. As is evident from the data shown in Figure 1, the induction of γH2AX by exposure to H2O2 was essentially limited to the DNA replicating cells. The cells initiating and terminating DNA replication [entering (enS) and exiting (exS) S phase] during the duration of the exposure to EdU had variable levels of EdU incorporation as their length of exposure to the precursor at the time of DNA replication varied between 1 and 120 min.
Among the cells that did not incorporate EdU, only a very few had γH2AX levels above that of the upper threshold of the H2O2-untreated cells (Fig. 1E, black dots). These were predominantly G2/M-phase cells which were previously shown to have high level of constitutive expression of γH2AX and activated ATM (8,9,22–24). The data also demonstrate that DNA replication was suppressed in cells exposed to the oxidant. This was reflected by a decreased level of EdU incorporation in the cells that were exposed to the precursor for 60 min in the absence of H2O2 and then for 60 min in the presence of H2O2 (Fig. 1C) compared with the cells exposed for the full 120 min to EdU in the absence of H2O2 (Fig. 1B). A relatively weak correlation (r = 0.36) was seen between the degree of EdU incorporation and extent of H2AX phosphorylation within the population of DNA replicating cells.
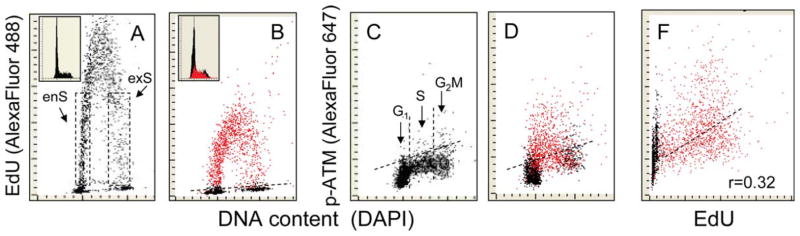
Essentially similar results were obtained in an experiment aimed to examine the relationship between DNA replication and activation of ATM in cells exposed to H2O2 (Fig. 2). Thus, activation of ATM was seen almost exclusively in cells that incorporated EdU. However, there were also cells that had incorporated EdU but did not show an increase in ATM activation (cells below the dashed line for ATM-S1981P in Fig. 2D). Among DNA replicating cells, the correlation between EdU incorporation and the degree of ATM activation (Ser1981 phosphorylation) was somewhat weaker (r = 0.32) that in the case of H2AX phosphorylation (r = 0.36).
Figure 2.
Correlation between DNA replication and phosphorylation of ATM on Ser1981 in A549 cells treated with H2O2. Panels A and B: the cells were exposed in culture to 5 μM EdU for 120 min. Panels B, D, and F: The cultures were initially treated with 5 μM EdU for 60 min, then exposed to 200 μM of H2O2 (still in the presence of EdU) for an additional 60 min. As in Fig. 1, using the “paint-a-gate” gating analysis, the EdU incorporating cells were colored red (B) and then data were replotted as ATM-S1981P vs. DNA content (D) or ATM-S1981P vs. EdU (F) bivariate distributions. It is apparent that predominantly DNA replicating (red) cells exhibited an increased level of ATM-S1081P following exposure to H2O2 [above the upper threshold for 97% of cells in the H2O2 untreated control culture (C, dashed line)]. A modest correlation between EdU incorporation and the induction of ATM-S1981P estimated for the population of DNA replicating (red colored) cells is apparent, with the coefficient (Pearson) r = 0.32 (F). The insets in panels A and B show DNA frequency histograms from the respective cultures. [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
The correlation between EdU incorporation versus induction of γH2AX (Fig. 1F) or versus induction of ATM-S1981P by H2O2 (Fig. 2F) included cells that were initiating (enS) and terminating (exS) DNA replication during the duration of the EdU pulse (120 min), i.e., were exposed to the precursor for variable (1–120 min) time intervals. To reveal whether there is a correlation between the DNA replication and the induction of γH2AX or ATM-S1981P in the cells that were exposed to EdU for the full 120 min, the paint-a-gate analysis was carried out as shown in Figure 3 to exclude the enS and exS cells. The data show a rather weak correlation between EdU incorporation and the induction of γH2AX (r = 0.21) and essentially no (very weak negative) correlation between EdU and the induction of ATM-S1981P(r = −13).
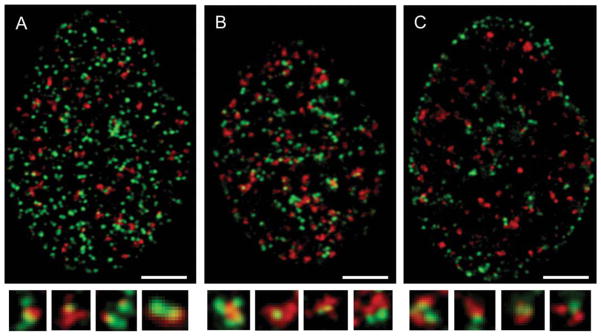
The intranuclear spatial relationship between the sites of DNA replication (“replication factories”) and the sites of H2AX phosphorylation (γH2AX foci) is shown in the confocal image of A549 nuclei. These cells were exposed to EdU for 30 min and subsequently treated with H2O2 for an additional 30 min (Fig. 4). The incorporated EdU was detected using the green-fluorophore (AlexaFluor 488)-tagged azide, while γH2AX was detected immunocytochemically using a secondary Ab tagged with a red fluorescing dye (AlexaFluor 568). The pattern of DNA replication sites (“replication factories”, green) of these nuclei is consistent with the early-S (A, B) and late-S (C) phase of the cycle. It is evident that there are numerous DNA replication sites alone, not associated with the γH2AX foci. It is also apparent that numerous γH2AX foci are also alone, not associated with the incorporated EdU. However, a significant proportion of the γH2AX foci are associated with the replication sites, and in fact, in several sites a distinct colocalization of EdU and γH2AX is apparent, revealed by yellow fluorescence, a result of the red plus green fluorescence overlap.
Figure 4.
Relationship between the sites of EdU incorporation and the induction of γH2AX foci in A549 cells treated with H2O2 Confocal images of A549 nuclei (selected equatorial planes) that were exposed to EdU for 30 min and then to H2O2 for additional 30 min. The incorporation of EdU was detected using the “Click-iT® methodology utilizing AlexaFluor 488-tagged azide (green fluorescence), whereas γH2AX was detected immunocytochemically with the secondary Ab labeled with AlexaFluor 568 (red fluorescence). The bottom panels show the sites of EdU incorporation that are in close proximity or colocalize with γH2AX foci, selected from the respective cell images (enlarged). The size marker = 5 μm.
Discussion
The present data confirm our previous reports that oxidative stress mediated by H2O2 induces phosphorylation of H2AX and activation of ATM (8,9,22–26). Although in the prior studies, we observed that these two DDR events were induced predominantly in S-phase cells, the present data provide more direct evidence of their association with actual DNA replication, this time revealed by the concurrent analysis of EdU incorporation. It is quite evident that almost all DNA replicating cells had levels of γH2AX elevated above that of the untreated cells (Fig. 1E). In the case of ATM activation, this relationship was somewhat less apparent in as much as a significant number of DNA replicating cells still had levels of ATM-S1981P similar to that of the S-phase cells from the control culture (below the dashed line in Figs. 2C and 2D).
A relatively low level of correlation was observed between the extent of EdU incorporation and expression of γH2AX (r = 0.36) or ATM-S1981P (r = 0.32). This was the case when all DNA-replicating cells, including those cells entering (enS) and exiting (exS) S phase during the EdU pulse were included. When the analysis was restricted to mid-S phase cells, (excluding the enS and exS cells as in Fig. 3), the correlation between EdU incorporation and γH2AX expression was still weaker (r = 0.21) and was not apparent for EdU versus ATM-S1981P. However, one has to be cautious in interpreting the data involving the relationship of these DDR events and the rate of DNA replication. The extent of incorporation of EdU may be affected by possible intercellular differences including: the uptake of the precursor, the size of the pool of endogenous thymidine competing with its analog EdU, as well as the rate of DNA replication.
The confocal images of the DNA replicating cells show the apparent association of some γH2AX foci with DNA replication factories, even to the point that some γH2AX foci colocalize with the DNA replication sites (Fig. 4). As it could be expected, there were sites of DNA replication that were not associated with γH2AX, likely reflecting replication through DNA sections having no H2O2-induced lesions It should be stressed, however, that a large proportion of the DNA replication sites were not associated with γH2AX foci. More extensive analysis of the association between the replication sites and induction of γH2AX by oxidative stress, including the analysis of DNA replicating cells at shorter pulses of exposure to EdU, different integrals (time windows) of the S-phase and statistical evaluation of the degree of the colocalization, is the subject of separate publication (manuscript in preparation).
Induction of distinct γH2AX foci is considered to report formation of DSBs (27,34,35). Our present data show that exposure to H2O2 induced γH2AX essentially only in DNA replicating cells and that some γH2AX foci either colocalized with or were in close proximity to DNA replication factories. These observations suggest the possibility that induction of DSBs occur as a result of the collapse of DNA replication forks upon encountering 8-oxo-dG, the primary DNA lesions caused by the oxidant. The role of replication stress and replication fork stalling and collapse induced by various agents including oxidants was recently reviewed by Allen et al. (36). It is more difficult to explain the presence of a large proportion of γH2AX foci alone, with no apparent association with DNA replication sites. It is possible that these γH2AX foci are the sites of DNA replication at which the amount of EdU incorporation at the moment of the collapse of the replication forks was as yet minimal and thus undetectable.
It was recently reported that oxidative stress induces activation of ATM directly, in the absence DNA, DSBs and the MRN complex (37,38). The authors postulate that in addition to its essential role in activation of the DSBs-induced signaling pathways, ATM may also serve as a sensor of oxidative stress and may activate other pathways related to oxidant-signaling. In these studies, activation of ATM by H2O2 in human primary fibroblasts was observed to occur with no evidence of H2AX phosphorylation. This is in contrast to the present findings and to our prior studies in which we observed induction of γH2AX upon treatment with H2O2 (8,9) or in response to other exogenous or endogenous oxidants (22–26). There is also extensive evidence in the literature concerning induction of γH2AX by different oxidants (39–45). Because H2AX phosphorylation induced by H2O2 occurs only in DNA replicating cells (Fig. 1), the apparent lack of H2AX phosphorylation upon treatment with H2O2 (37) may indicate that the cells were in a stationary phase of growth, in the G0/1 phase. It is also possible that primary fibroblasts have a different response to oxidants in terms of H2AX phosphorylation than the pulmonary carcinoma A549 cells or other tumor lines.
Supplementary Material
Acknowledgments
Grant sponsor: NCI; Grant number: CA RO1 28 704; Grant sponsor: Jagiellonian University (DS, BW).
Footnotes
Additional Supporting Information (MIFlowCyt item location) may be found in the online version of this article.
Literature Cited
- 1.Beckman BK, Ames BN. The free radical theory of aging matures. Phys Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 2.Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:13300–13305. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 3.Gorbunova V, Seluanov A. Making ends meet in old age: DSB repair and aging. Mech Ageing Dev. 2005;126:621–628. doi: 10.1016/j.mad.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Karanjawala ZE, Lieber MR. DNA damage and aging. Mech Ageing Dev. 2004;125:405–416. doi: 10.1016/j.mad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Extension of murine life span by over expression of catalase targeted to mitochondria. Science. 2005;308:1875–1878. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 7.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA. 2003;100:12871–12876. doi: 10.1073/pnas.2135498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Tanaka T, Halicka HD, Traganos F, Zarebski M, Dobrucki J, Darzynkiewicz Z. Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports the aging-related processes. Cytometry Part A. 2007;71A:905–914. doi: 10.1002/cyto.a.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka T, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle. 2006;5:1940–1945. doi: 10.4161/cc.5.17.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T, Halicka HD, Traganos F, Darzynkiewicz Z. Phosphorylation of histone H2AX on Ser 139 and activation of ATM during oxidative burst in phorbol ester-treated human leukocytes. Cell Cycle. 2006;5:2671–2675. doi: 10.4161/cc.5.22.3472. [DOI] [PubMed] [Google Scholar]
- 11.Shacter E, Beecham EJ, Covey JM, Kohn KW, Potter M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis. 1988;9:2297–2304. doi: 10.1093/carcin/9.12.2297. [DOI] [PubMed] [Google Scholar]
- 12.Chong YC, Heppner GH, Paul LA, Fulton AM. Macrophage-mediated induction of DNA strand breaks in target tumor cells. Cancer Res. 1989;49:6652–6657. [PubMed] [Google Scholar]
- 13.Taioli E, Sram RJ, Garte BM, Kalina I, Popov TA, Farmer PB. Effects of polycyclic aromatic hydrocarbons (PAHs) in environmental pollution on exogenous and oxidative DNA damage (EXPAH project): Description of the population under study. Mutat Res. 2007;620:1–6. doi: 10.1016/j.mrfmmm.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Demirbag R, Yilmaz R, Kocyigit A, Guzel S. Effect of coronary angiography on oxidative DNA damage observed in circulating lymphocytes. Angiology. 2007;58:141–147. doi: 10.1177/0003319707300547. [DOI] [PubMed] [Google Scholar]
- 15.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Hydroxyl radicals and DNA base damage. Mutat Res. 1999;424:9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 16.Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–222. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- 17.Pastwa E, Blasiak J. Non-homologous DNA end joining. Acta Biochim Pol. 2003;50:891–908. [PubMed] [Google Scholar]
- 18.Jeggo PA, Lobrich M. Artemis links ATM to double strand end rejoining. Cell Cycle. 2005;4:359–362. doi: 10.4161/cc.4.3.1527. [DOI] [PubMed] [Google Scholar]
- 19.Blagosklonny MV. Aging and immortality: Quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–2102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- 20.Blagosklonny MV. Paradoxes of aging. Cell Cycle. 2007;6:2997–3003. doi: 10.4161/cc.6.24.5124. [DOI] [PubMed] [Google Scholar]
- 21.Mallette FA, Ferbeyre G. The DNA damage signaling pathway connects oncogenic stress to cellular senescence. Cell Cycle. 2007;6:1831–1836. doi: 10.4161/cc.6.15.4516. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Kurose A, Halicka HD, Traganos F, Darzynkiewicz Z. 2-Deoxy-D-glucose reduces the level of constitutive activation of ATM and phosphorylation of histone H2AX. Cell Cycle. 2006;5:878–882. doi: 10.4161/cc.5.8.2681. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T, Kajstura M, Halicka HD, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation are strongly amplified during mitogenic stimulation of lymphocytes. Cell Prolif. 2007;40:1–13. doi: 10.1111/j.1365-2184.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Tanaka T, Kurose A, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation on Ser-139 in cells untreated by genotoxic agents is cell-cycle phase specific and attenuated by scavenging reactive oxygen species. Int J Oncol. 2006;29:495–501. [PubMed] [Google Scholar]
- 25.Tanaka T, Halicka HD, Traganos F, Darzynkiewicz Z. Phosphorylation of histone H2AX on Ser 139 and activation of ATM during oxidative burst in phorbol ester-treated human leukocytes. Cell Cycle. 2006;5:2671–2675. doi: 10.4161/cc.5.22.3472. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Kurose A, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Nitrogen oxide-releasing aspirin induces histone H2AX phosphorylation, ATM activation, and apoptosis preferentially in S-phase cells; involvement of reactive oxygen species. Cell Cycle. 2006;5:1669–1674. doi: 10.4161/cc.5.15.3100. [DOI] [PubMed] [Google Scholar]
- 27.Sedelnikova OA, Rogakou EP, Panuytin IG, Bonner W. Quantitive detection of 125IUdr-induced DNA double-strand breaks with γ-H2AX antibody. Radiat Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darzynkiewicz Z, Traganos F, Zhao H, Halicka HD, Li J. Cytometry of DNA replication and RNA synthesis: Historical perspective and recent advances based on “click chemistry”. Cytometry Part A. 2011;79A:328–337. doi: 10.1002/cyto.a.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diermeier-Daucher S, Clarke ST, Hill D, Vollmann-Zwerenz A, Bradford JA, Brockhoff G. Cell type specific applicability of 5-ethynyl-2-deoxyuridine (EdU) for dynamic proliferation assessment in flow cytometry. Cytometry Part A. 2009;75A:535–546. doi: 10.1002/cyto.a.20712. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H, Li J, Traganos F, Halicka HD, Zarebski M, Dobrucki J, Darzynkiewicz Z. Cell fixation in zinc salt solution (ZBF) is compatible with DNA damage response detection by phospho-specific antibodies. Cytometry Part A. 2011;79A:470–476. doi: 10.1002/cyto.a.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Traganos F, Darzynkiewicz Z. Kinetics of the UV-induced DNA damage response in relation to cell cycle phase. Correlation with DNA replication. Cytometry Part A. 2010;77A:285–293. doi: 10.1002/cyto.a.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darzynkiewicz Z, Bedner E, Gorczyca W, Melamed MR. Laser scanning cytometry. A new instrumentation with many applications. Exp Cell Res. 1999;249:1–12. doi: 10.1006/excr.1999.4477. [DOI] [PubMed] [Google Scholar]
- 34.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 36.Allen C, Ashley AK, Hromas R, Nickoloff JA. More forks on the road to replication stress recovery. J Mol Cell Biol. 2011;3:4–12. doi: 10.1093/jmcb/mjq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Z, Kozlow S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 38.Guo Z, Deshpande R, Paull TT. ATM activation in the presence of oxidative stress. Cell Cycle. 2010;9:4805–4811. doi: 10.4161/cc.9.24.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartkova J, Bakkenist CJ, Rajpert-De Meyts E, Skakkebaek NE, Sehested M, Lukas J, Kastan MB, Bartek J. ATM activation in normal human tissues and testicular cancer. Cell Cycle. 2005;4:838–845. doi: 10.4161/cc.4.6.1742. [DOI] [PubMed] [Google Scholar]
- 40.Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem. 2003;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Yang J, Huang H. Oxidative stress induced H2AX phosphorylation in human spermatozoa. FEBS Letters. 2006;580:6161–6168. doi: 10.1016/j.febslet.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Zhang HY, Hormi-Carver K, Zhang X, Spechler SJ, Souza RF. In benign Barrett’s epithelial cells, acid exposure generates reactive oxygen species that cause DNA double-strand breaks. Cancer Res. 2009;69:9083–9089. doi: 10.1158/0008-5472.CAN-09-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartkova J, Hamerlik P, Stockhausen MT, Ehrmann J, Hlobikova A, Laursen H, Kalita O, Kolar Z, Paulsen HS, Broholm H, Lukas J, Bartek J. Replication stress and oxidative DNA damage contribute to aberrant constitutive activation of DNA damage signaling in human glioma. Oncogene. 2010;29:5095–5102. doi: 10.1038/onc.2010.249. [DOI] [PubMed] [Google Scholar]
- 44.Braidy N, Guillemin GJ, Mansour H, Chen-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in Wistar rats. PLoS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Diestel A, Drescher C, Miera O, Berger F, Schmitt KRL. Hypothermia protects H9c2 cardiomyocytes from H2O2 induced apoptosis. Cryobiology. 2011;62:53–61. doi: 10.1016/j.cryobiol.2010.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.