Abstract
The gross majority of classical apoptotic hallmarks can be rapidly examined by multiparameter flow cytometry. As a result, cytometry became a technology of choice in diverse studies of cellular demise. In this context, a novel class of substituted unsymmetrical cyanine SYTO probes has recently become commercially available. Derived from thiazole orange, SYTO display low intrinsic fluorescence, with strong enhancement upon binding to DNA and/or RNA. Broad selection of excitation/emission spectra has recently driven implementation of SYTO dyes in polychromatic protocols with the detection of apoptosis being one of the most prominent applications In this chapter, we outline a handful of commonly used protocols for the assessment of apoptotic events using selected SYTO probes (SYTO 16, 62, 80) in conjunction with common plasma membrane permeability markers (PI, YO-PRO 1, 7-AAD).
Keywords: Flow cytometry, Apoptosis, Lymphoma, SYTO 16, SYTO 62, SYTO 80
1. Introduction
During the past decade, mechanisms underlying cell death have entered into a focus of many researchers in diverse fields of biomedicine. The gross majority of classical apoptotic hallmarks can be rapidly examined by multiparameter flow cytometry (1). As a result, cytometry became a technology of choice in diverse studies of cellular demise, and diverse cytometric assays have been introduced (1–3). To date, however, live-cell assays that are based on cell permeant DNA probes suffered mostly from their unfavorable spectral characteristics that necessitate UV excitation source and dedicated optics. Excessive toxicity/phototoxicity precluded also long-term studies such as cell sorting with subsequent cell cultivation (4, 5).
In this context, a novel class of substituted unsymmetrical cyanine SYTO probes has recently become commercially available. Derived from thiazole orange, SYTO display low intrinsic fluorescence, with strong enhancement upon binding to DNA and/or RNA. This novel class of probes spans a broad range of visible excitation and emission spectra: (1) SYTO blue (Ex/Em 419–452/445–484 nm); (2) SYTO green (Ex/Em 483–521/500–556 nm); (3) SYTO orange (Ex/Em 528–567/544–583 nm); and (4) SYTO red (Ex/Em 598–654/620–680 nm) (4, 6–8). Exploitation of SYTO probes to cytometric detection of apoptosis is a relatively new method (4, 6–8). This methodology, however, is slowly gaining appreciation as an easy to perform, live-cell assay (6–8). Importantly, we have recently showed that cyanine SYTO dyes represent a promising class of inert probes that do not adversely affect normal cellular physiology (9). When preloaded or continuously present in medium, SYTO do not interfere with cell viability, and their intracellular retention permits straightforward and kinetic analysis of investigational drug/compound cytotoxicity (9). Reduction of sample processing achieved with these protocols is important for preservation of fragile apoptotic cells. SYTO 16-based live cell sorting represents also a novel approach to supravitally track progression of apoptotic cascade in response to investigational anticancer agents (9).
In this chapter, we outline a handful of commonly used protocols for the assessment of apoptotic events using selected SYTO probes (SYTO 16, 62, 80) in conjunction with common plasma membrane permeability markers (PI, YO-PRO 1, 7-AAD).
2. Materials
2.1. Detection of Apoptosis Using SYTO 16 and Propidium Iodide
Cell suspension (2.5 × 105–2 × 106 cells/mL).
1× PBS.
1 mM SYTO 16 stock solution in DMSO. Store protected from light at −20°C. Stable for over 12 months. Caution: although there are no reports on SYTO 16 toxicity, appropriate precautions should always be applied when handling SYTO 16 solutions.
10 μM SYTO 16 working solution in PBS (prepare fresh as required).
50 μg/mL propidium iodide (PI) stock solution in PBS. Store protected from light at +4°C. Stable for over 12 months. Caution: PI is a DNA binding molecule and thus can be considered as a potential carcinogen. Always handle with care and use protective gloves.
30 mM Verapamil in EtOH (P-gp inhibitor). Store protected from light at −20°C. Stable for over 12 months.
1.5 mL Eppendorf tubes.
12 × 75 mm polystyrene FACS tubes.
2.2. Detection of Apoptosis Using SYTO 62 and YO-PRO 1
Cell suspension (2.5 × 105–2 × 106 cells/mL).
1× PBS.
1 mM SYTO 62 stock solution in DMSO. Store protected from light at −20°C. Stable for over 12 months. Caution: although there are no reports on SYTO 62 toxicity, appropriate precautions should always be applied when handling SYTO 62 solutions.
10 μM SYTO 62 working solution in PBS (prepare fresh as required).
1 mM YO-PRO 1 stock solution in DMSO. Store protected from light at −20°C. Stable for over 12 months. Caution: although there are no reports on YO-PRO 1 toxicity, appropriate precautions should always be applied when handling YO-PRO 1 solutions.
10 μM YO-PRO 1 working solution in PBS (prepare fresh as required).
30 mM Verapamil in EtOH (P-gp inhibitor). Store protected from light at −20°C. Stable for over 12 months.
1.5 mL Eppendorf tubes.
12 × 75 mm polystyrene FACS tubes.
2.3. Detection of Apoptosis Using SYTO 80 and 7-AAD
Cell suspension (2.5 × 105–2 × 106 cells/mL).
1× PBS.
1 mM SYTO 80 stock solution in DMSO. Store protected from light at −20°C. Stable for over 12 months. Caution: although there are no reports on SYTO 80 toxicity, appropriate precautions should always be applied when handling SYTO 80 solutions.
10 μM SYTO 80 working solution in PBS (prepare fresh as required).
50 μg/mL 7-aminoactinomycin D (7-AAD) stock solution in PBS. Store protected from light at +4°C. Stable for over 12 months. Caution: 7-AAD is a DNA binding molecule and thus can be considered as a potential carcinogen. Always handle with care and use protective gloves.
30 mM Verapamil in EtOH (P-gp inhibitor). Store protected from light at −20°C. Stable for over 12 months.
1.5 mL Eppendorf tubes.
12 × 75 mm polystyrene FACS tubes.
3. Methods
3.1. Detection of Apoptosis Using SYTO 16 and PI
The cytometric detection of SYTO 16 fluorescence loss is a sensitive marker of early apoptotic events (Fig. 1a; see Notes 1–3) (4, 7, 9). The following protocol describes a combined use of SYTO 16 (Ex/Em 488/518 nm) together with a marker of plasma membrane integrity: propidium iodide (PI; Ex/Em 488/575–670 nm). Every assay allows for sensitive discrimination between viable, early apoptotic, and late apoptotic/necrotic subpopulations based on differential SYTO staining profiles (Fig. 1a; see Notes 3–5) (4, 9). The method presented below is a single-step and time-saving assay. Minimized washing steps permit maintenance of fragile apoptosing population in intact state (see Note 5) (4, 7).
Fig. 1.
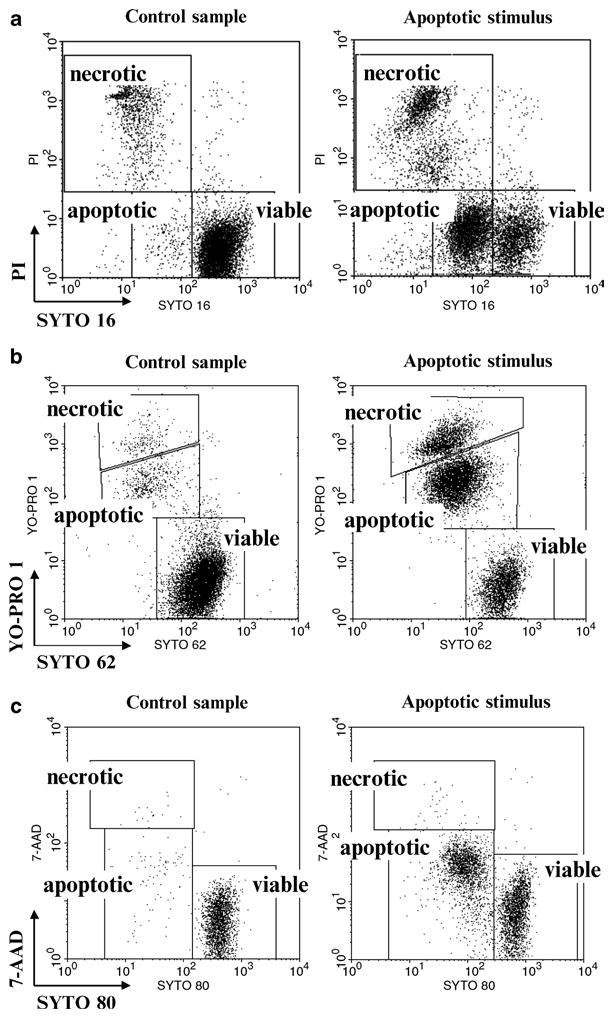
Discrimination of viable, apoptotic, and late apoptotic/necrotic cells using selected SYTO probes and plasma membrane permeability markers: (a) SYTO 16 probe in conjunction with plasma membrane permeability marker pro-pidium iodide (PI). Both probes were excited using 488 nm laser. SYTO 16 and PI fluorescence signals were logarithmically amplified using 530 and 575 nm band-pass filters, respectively. Debris were excluded electronically. Analysis based on bivariate dot plots SYTO 16 vs. PI is shown. (b) SYTO 62 probe in conjunction with plasma membrane permeability marker YO-PRO 1. Probes were excited using 633 and 488 nm lasers, respectively. Logarithmically amplified fluorescence signals were collected using 660 and 530 nm band-pass filters, respectively. Debris were excluded electronically. (c) SYTO 80 probe in conjunction with plasma membrane permeability marker 7-aminoactinomycin D (7-AAD). Both probes were excited using 488 nm laser. SYTO 80 and 7-AAD fluorescence signals were logarithmically amplified using 575 and 677 nm band-pass filters, respectively. Debris were excluded electronically.
Collect cell suspension into 12 × 75-mm Falcon FACS tube and centrifuge for 5 min, 243 × g at room temperature (RT).
Prepare staining mixture by adding 875 μL of PBS, 25 μL of 10 μM SYTO 16, and 100 μL of 50 μg/mL PI (final concentration 250 nM SYTO 16 and 5 μg/mL PI).
Add 1 μL of 30 mM Verapamil to the staining mixture (final concentration 30 μM; see Note 6).
Discard supernatant and gently resuspend cells in 100 μL of staining mixture.
Incubate for 15 min at RT.
Add 500 μL of PBS.
Analyze on flow cytometer with 488 nm excitation line (Argon-ion laser or solid-state laser) with emissions collected at 530 nm (SYTO 16) and 575–610 nm (PI). Adjust the logarithmic amplification scale to distinguish between viable cells (bright SYTO 16+/PI−), early apoptotic cells (dim SYTO 16+/PI−), and late apoptotic and/or necrotic cells with compromised plasma membranes (low SYTO 16+/PI+) (Fig. 1a; see Notes 7–9).
3.2. Detection of Apoptosis Using SYTO 62 and YO-PRO 1
The principle of this assay is similar to the above described SYTO 16 protocol (see Notes 1–3). The main advantage of this protocol is the minimal interbeam compensation required between SYTO 62 and YO-PRO 1 (see Note 10) (4, 8). Moreover, the use of YO-PRO 1 (Ex/Em 491/509 nm) as a marker of plasma membrane integrity allows for a more sensitive detection of early apoptotic cells than propidium iodide (8, 10). Furthermore, other channels from 488 nm excitation line are free to combine this protocol with, e.g., immunophenotyping.
Collect cell suspension into 12 × 75 mm Falcon FACS tube and centrifuge for 5 min, 1,100 rpm at room temperature (RT).
Prepare staining mixture by adding 970 μL of PBS, 5 μL of 10 μM SYTO 62, and 25 μL of 10 μM YO-PRO 1 (final concentration 50 nM SYTO 62 and 250 nM YO-PRO 1).
Add 1 μL of 30 mM Verapamil to the staining mixture (final concentration 30 μM; see Note 6).
Discard the supernatant and gently resuspend cells in 100 μL of staining mixture.
Incubate for 15 min at RT.
Add 500 μL of PBS.
Analyze on a flow cytometer with 488 nm (Argon-ion laser or solid-state laser) and 633/635 nm excitation lines. Emissions should be collected at 530 nm (YO-PRO 1) and 660 nm (SYTO 62) (see Note 10). Adjust the logarithmic amplification scale to distinguish between viable cells (bright SYTO 62+/YO-PRO 1−), early apoptotic cells (dim SYTO 62+/dim YO-PRO 1+), and late apoptotic and/or necrotic cells with compromised plasma membranes (low SYTO 62+/bright YO-PRO 1+) (Fig. 1b; see Note 10).
3.3. Detection of Apoptosis Using SYTO 80 and 7-AAD
This assay utilizes a new orange fluorescent probe SYTO 80, and its principle is similar to the above described SYTO 16 and SYTO 62 protocols (see Notes 1–3) (4). The main advantage of this protocol is that channels from other excitation lines, e.g., 633 or 405 nm are free to combine this protocol with, e.g., immunophenotyping.
Collect cell suspension into 12 × 75-mm Falcon FACS tube and centrifuge for 5 min, 1,100 rpm at room temperature (RT).
Prepare staining mixture by adding 875 μL of PBS, 25 μL of 10 μM SYTO 80, and 100 μL of 50 μg/mL 7-AAD (final concentration 250 nM SYTO 80 and 5 μg/mL 7-AAD).
Add 1 μL of 30 mM Verapamil to the staining mixture (final concentration 30 μM; see Note 6).
Discard the supernatant and gently resuspend cells in 100 μL of staining mixture.
Incubate for 15 min at RT.
Add 500 μL of PBS.
Analyze on flow cytometer with 488 nm excitation line (Argon-ion laser or solid-state laser) with emissions collected at 575–610 nm (SYTO 80) and ≥670 nm (7-AAD). Adjust the logarithmic amplification scale to distinguish between viable cells (bright SYTO 80+/7-AAD−), early apoptotic cells (dim SYTO 80+/dim 7-AAD+), and late apoptotic and/or necrotic cells with compromised plasma membranes (low SYTO 80+/bright 7-AAD+) (Fig. 1c).
Acknowledgments
Supported by BBSRC, EPSRC, and Scottish Funding Council, funded under RASOR (DW); NCI CA RO1 28 704 (ZD). JS received the L’Oreal Poland-UNESCO “For Women In Science” 2007 Award. Views and opinions described in this chapter were not influenced by any conflicting commercial interests.
Footnotes
The universal term “apoptosis,” has a propensity to misinterpret the actual phenotype of cell suicide program (1, 11–13). Thus, the use of the generic term apoptosis should be always accompanied by listing the particular morphological and/or biochemical apoptosis-associated feature(s) that was (were) detected (1–3).
Morphological criteria (examined by the light, fluorescent, and electron microscopy) are still the “gold standard” to define the mode of cell death and confirm results obtained by flow cytometry (1–4). Lack of microscopic examination may potentially lead to the misclassification and false positive or negative artifacts, and is a common drawback of the experimental design (1–4). The best example of such misclassification is identification of phagocytes that engulfed apoptotic bodies as individual apoptotic cells (3).
Following initiation of caspase-dependent apoptosis, cells loaded with selected SYTO stains exhibit gradual reduction in fluorescence signal intensity to dim values. This phenomenon substantially precedes plasma membrane permeability changes (4, 6, 7). Evidence from recently published data indicates an overall higher sensitivity of SYTO probes in the detection of early apoptotic events as compared to Annexin V-based assays (4, 7, 8). When progression toward the terminal stages of cellular demise advances, loss of SYTO fluorescence intensifies, and this usually coincides with the increased plasma membrane permeability to PI and 7-AAD (4, 7, 8).
We have recently shown that SYTO 16 allows discrimination between primary and secondary necrotic cells (7). Therefore, SYTO 16 provides substantial enhancement over the standard PI exclusion assay in discerning cell demise mode by flow cytometry (7).
Importantly, SYTO probes prove in many instances inert and safe for tracking cells over extended periods of time which may open up new opportunities for single-cell analysis protocols by both fluorescent activated cell sorting (FACS) and Lab-on-a-Chip platforms (9).
Recent noteworthy reports provided strong evidence that at least some SYTO probes can be substrates for MDR efflux pumps (e.g., P-glycoprotein; P-gp) (14, 15). Caution should be, thus, exercised when using SYTO probes in cells with active ABC-class transporters. It is always advisable to confirm MDR status of studied cell population. In cells with active P-gp, its inhibition (e.g., by Verapamil hydrochloride, PSC833, Cyclosporin A) is required to avoid masking of apoptotic SYTOdim subpopulation by SYTOdim subpopulation engendered by an active dye efflux (14, 15). Truly apoptotic reduction of SYTO fluorescence to dim values is not affected by the presence of P-gp inhibitors (14).
Both fluorophores are easily excited by 488 nm lasers. While propidium iodide can be detected in both 575–610 and >650 nm channels, SYTO 16 is detected using standard fluoroscein (FITC) band-pass filter at 530 nm. Due to the significant spectral overlap of SYTO16 fluorescence in 575–610 nm channels, special attention is required to apply proper compensation between both channels. Adjust the logarithmic amplification scale to distinguish between viable cells (bright SYTO 16+/PI− events), apoptotic cells (dim SYTO 16+/PI− events), and late apoptotic/necrotic cells with compromised plasma membranes (SYTO 16−/PI+ events) as presented in Fig. 1a. In every cell system, there is a need to optimize concentration/incubation time of SYTO 16 probe as well as PMT voltage to achieve maximal resolution between bright, dim, and low/negative SYTO 16 events (7–9).
One should always bear in mind that results obtained using SYTO-based assays may vary when compared to assays detecting different cellular processes. Results acquired with SYTO probes should, therefore, never be considered conclusive without verification by independent methods (4).
Some apoptotic markers (e.g., loss of SYTO fluorescence to dim values; Annexin V immunoreactivity or oligonucleosomal DNA fragmentation) may not be detected in specimens challenged with divergent stimuli or microenvironmental conditions (e.g., cytokines, growth factor deprivation, heterotypic cell culture, etc.). It is always advisable to simultaneously study several markers to provide a multidimensional view of advancing apoptotic cascade (1–4). Multiparameter assays detecting several cell attributes are the most desirable solution for flow cytometric quantification of apoptosis (1–4).
SYTO 62 fluorescence can be conveniently detected using a standard allophycocyanin (APC) 660 nm band-pass filter. Use of SYTO 62 and YO-PRO 1 requires minimal interbeam compensation. Similar to SYTO 16, SYTO 62 dim (deemed apoptotic) events will dimly stain with YO-PRO 1 as a result of early loss in the plasma membrane function. Adjust the logarithmic amplification scale to distinguish between viable cells (bright SYTO 62+/YO-PRO 1−), early apoptotic cells (dim SYTO 62+/dim YO-PRO 1+), and late apoptotic and/or necrotic cells with compromised plasma membranes (low SYTO 62+/bright YO-PRO 1+) (8).
References
- 1.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 2.Darzynkiewicz Z, Li X, Bedner E. Use of flow and laser-scanning cytometry in analysis of cell death. Methods Cell Biol. 2001;66:69–109. doi: 10.1016/s0091-679x(01)66005-9. [DOI] [PubMed] [Google Scholar]
- 3.Darzynkiewicz Z, Huang X, Okafuji M, King MA. Cytometric methods to detect apoptosis. Methods Cell Biol. 2004;75:307–41. doi: 10.1016/s0091-679x(04)75012-8. [DOI] [PubMed] [Google Scholar]
- 4.Wlodkowic D, Skommer J, Darzynkiewicz Z. SYTO probes in the cytometry of tumor cell death. Cytometry A. 2008;73:496–507. doi: 10.1002/cyto.a.20535. [DOI] [PubMed] [Google Scholar]
- 5.Wlodkowic D, Darzynkiewicz Z. Please do not disturb: destruction of chromatin structure by supravital nucleic acid probes revealed by a novel assay of DNA-histone interaction. Cytometry A. 2008;73:877–879. doi: 10.1002/cyto.a.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey T. Nucleic acid dyes for detection of apoptosis in live cells. Cytometry. 1995;21:265–74. doi: 10.1002/cyto.990210307. [DOI] [PubMed] [Google Scholar]
- 7.Wlodkowic D, Skommer J, Pelkonen J. Towards an understanding of apoptosis detection by SYTO dyes. Cytometry A. 2007;71:61–72. doi: 10.1002/cyto.a.20366. [DOI] [PubMed] [Google Scholar]
- 8.Wlodkowic D, Skommer J, Hillier C, Darzynkiewicz Z. Multiparameter detection of apoptosis using red-excitable SYTO probes. Cytometry A. 2008;73:563–569. doi: 10.1002/cyto.a.20564. [DOI] [PubMed] [Google Scholar]
- 9.Wlodkowic D, Skommer J, Faley S, Darzynkiewicz Z, Cooper JM. Dynamic analysis of apoptosis using cyanine SYTO probes: from classical to microfluidic cytometry. Exp Cell Res. 2009;315:1706–14. doi: 10.1016/j.yexcr.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idziorek T, Estaquier J, De Bels F, Ameisen JC. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods. 1995;185:249–58. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 11.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–98. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 12.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–30. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 13.Blagosklonny MV. Cell death beyond apoptosis. Leukemia. 2000;14:1502–1508. doi: 10.1038/sj.leu.2401864. [DOI] [PubMed] [Google Scholar]
- 14.Schuurhuis GJ, Muijen MM, Oberink JW, de Boer F, Ossenkoppele GJ, Broxterman HJ. Large populations of non-clonogenic early apoptotic CD34-positive cells are present in frozen-thawed peripheral blood stem cell transplants. Bone Marrow Transplant. 2001;27:487–98. doi: 10.1038/sj.bmt.1702809. [DOI] [PubMed] [Google Scholar]
- 15.van der Pol MA, Broxterman HJ, Westra G, Ossenkoppele GJ, Schuurhuis GJ. Novel multiparameter flow cytometry assay using Syto16 for the simultaneous detection of early apoptosis and apoptosis-corrected P-glycoprotein function in clinical samples. Cytometry B. 2003;55:14–21. doi: 10.1002/cyto.b.10024. [DOI] [PubMed] [Google Scholar]


