Abstract
Type 2 diabetes mellitus is likely the third modifiable risk factor for pancreatic cancer after cigarette smoking and obesity. Epidemiological investigations have found that long-term type 2 diabetes mellitus is associated with a 1.5- to 2.0-fold increase in the risk of pancreatic cancer. A causal relationship between diabetes and pancreatic cancer is also supported by findings from prediagnostic evaluations of glucose and insulin levels in prospective studies. Insulin resistance and associated hyperglycemia, hyperinsulinemia, and inflammation have been suggested to be the underlying mechanisms contributing to development of diabetes-associated pancreatic cancer. Signaling pathways that regulate the metabolic process also play important roles in cell proliferation and tumor growth. Use of the antidiabetic drug metformin has been associated with reduced risk of pancreatic cancer in diabetics and recognized as an antitumor agent with the potential to prevent and treat this cancer. On the other hand, new-onset diabetes may indicate subclinical pancreatic cancer, and patients with new-onset diabetes may constitute a population in whom pancreatic cancer can be detected early. Biomarkers that help define high-risk individuals for clinical screening for pancreatic cancer are urgently needed. Why pancreatic cancer causes diabetes and how diabetes affects the clinical outcome of pancreatic cancer have yet to be fully determined. Improved understanding of the pathological mechanisms shared by diabetes and pancreatic cancer would be the key to the development of novel preventive and therapeutic strategies for this cancer.
Keywords: diabetes, pancreatic cancer, epidemiology
INTRODUCTION
As a consequence of the obesity epidemic, the incidence of diabetes is increasing globally, with an estimated 285 million people, or 6.6% of the population from 20 to 79 years of age, affected. Type 2 diabetes mellitus accounts for more than 95% of the cases. Type 2 diabetes mellitus has been associated with an increased risk of several human cancers, such as liver, pancreatic, endometrial, colorectal, breast, and bladder cancer. After cigarette smoking and obesity, type 2 diabetes mellitus is likely the third modifiable risk factor for pancreatic cancer. However, the relationship between diabetes and pancreatic cancer is complex and intertwined. On one hand, diabetes can be an early manifestation of pancreatic cancer. On the other, diabetes has been implicated as a predisposing factor for pancreatic cancer [1-3]. An improved understanding of the association between diabetes and pancreatic cancer and the mechanism underlying this association would aid the development of novel strategies for the prevention and treatment of this cancer.
Epidemiological Evidence of Diabetes as a Risk Factor for Pancreatic Cancer
Diabetes or impaired glucose tolerance is present in 50-80% of patients with pancreatic cancer [4-6]. More than 85% of the diabetes cases in patients with pancreatic cancer are diagnosed fewer than 2 years before the cancer diagnosis or during the cancer course. Patients with new-onset diabetes, in other words, those in whom diabetes was diagnosed no more than 2 years before cancer was diagnosed, are usually considered as having “secondary diabetes” caused by the cancer under the assumption that pancreatic cancer is a rapidly fatal disease: a person with pancreatic cancer-caused diabetes would not live for many years without his or her cancer being diagnosed. However, most cancers have long latencies, and many diabetes and prediabetic cases are undiagnosed before the cancer is diagnosed. No current clinical or laboratory methods accurately determine the time when disease is initiated or distinguish the type II diabetes from the pancreatic cancer-caused diabetes. Therefore, misclassification bias seems to be unavoidable in studies of the association between diabetes and pancreatic cancer.
To date, more than 20 case-control studies [7-25] and cohort and nested case-control studies [26-45] with information on the association between diabetes and pancreatic cancer have been reported. Most of these studies were included in two meta-analyses investigating the risk of pancreatic cancer in relation to diabetes [46,47]. The first meta-analysis, conducted in 1995, included 20 of these 40 published case-control and cohort studies and reported an overall estimated relative risk (RR) of pancreatic cancer of 2.1 with a 95% confidence interval (CI) of 1.6-2.8. These values were relatively unchanged when the analyses were restricted to patients who had diabetes for at least 5 years (RR, 2.0 [95% CI, 1.2-3.2]) [46]. The second meta-analysis, which was conducted in 2005, included 17 case-control and 19 cohort and nested case-control studies published from 1996 to 2005 and demonstrated an overall odds ratio (OR) for pancreatic cancer of 1.8 and 95% CI of 1.7-1.9 [47]. Individuals diagnosed with diabetes within 4 years before their pancreatic cancer diagnosis had a 50% greater risk of pancreatic cancer than did those diagnosed with diabetes more than 5 years before their cancer diagnosis (OR, 2.1 [95% CI, 1.9-2.3] versus OR, 1.5 [95% CI, 1.3-1.8]; P = 0.005). In a recent pooled analysis of 2192 patients with pancreatic cancer and 5113 cancer-free controls in three large case-control studies conducted in the United States (results of two of the three studies were published after 2005), diabetes was associated with a 1.8-fold increase in risk of pancreatic cancer (95% CI, 1.5-2.1). Risk estimates decreased as the number of years with diabetes increased. Individuals with diabetes for 2 or fewer, 3-5, 6-10, 11-15, or more than 15 years had ORs (95% CIs) of 2.9 (2.1-3.9), 1.9 (1.3-2.6), 1.6 (1.2-2.3), 1.3 (0.9-2.0), and 1.4 (1.0-2.0), respectively (P < 0.0001 for trend) [48]. These results support a modest association between type 2 diabetes mellitus and pancreatic cancer.
In general, risk estimates for diabetes-associated pancreatic cancer were higher in individuals with short durations of diabetes than in those with long durations. This inverse association between duration of diabetes and risk of pancreatic cancer may be explained by two hypotheses. First, the increased risk of pancreatic cancer in individuals with short diabetes durations (≤2 years) resulted primarily from reverse causality. Second, the decreasing risk of pancreatic cancer with increasing duration of diabetes may have been partially related to lifestyle changes after diabetes diagnosis or use of certain antidiabetic medications. At present, little available evidence supports or refutes these hypotheses. Because cardiovascular complications were much more common in diabetic patients than in nondiabetic patients in these studies, the risk of pancreatic cancer in patients with long-term diabetes may be underestimated because of the death of diabetic patients not having pancreatic cancer diagnoses.
Whereas cigarette smoking and obesity are established risk factors for both type 2 diabetes mellitus and pancreatic cancer, whether diabetes is an independent risk factor for pancreatic cancer remains questionable. Limited by the sample sizes in individual studies, this question was not examined in most published reports. In the analysis of pooled data from the three large case-control studies described above, the researchers did not observe significant interaction between diabetes and sex, race, education, smoking, alcohol, or body mass index (BMI) (P = 0.28, 0.52, 0.22, 0.95, 0.36, and 0.35, respectively, for interaction terms) [48]. Investigators observed comparable risk estimates among nonsmokers (OR, 2.2 [95% CI, 1.7-2.7]), former smokers (OR, 2.1 [95% CI, 1.7-2.6]), and current smokers (OR, 2.0 [95% CI, 1.4-2.8]) and between individuals with BMIs of no more than 25 kg/m2 (OR, 1.9 [95% CI, 1.5-2.4]) and greater than 25 kg/m2 (OR, 2.1 [95% CI, 1.6-2.6]). These observations support an independent association between diabetes and pancreatic cancer regardless of smoking status or BMI. Additional large studies are required to confirm these findings and further examine the impact of diabetes on the associations between pancreatic cancer and other known or suspected risk factors, such as a family history of cancer and dietary factors.
The relationship between long-standing diabetes and risk of pancreatic cancer is also supported by results from biomarker studies. One prospective study directly examined the relationship between prediagnostic serum insulin levels and pancreatic cancer risk, demonstrating a twofold increase in risk after excluding cases diagnosed in the first 5 years of follow-up (RR, 2.01 [95% CI, 1.03-3.93] for the highest versus lowest quartile insulin level) [49]. Also, a meta-analysis of studies of cancers of the colon and rectum, pancreas, breast, and endometrium showed an excessively high risk of colorectal and pancreatic cancer associated with high levels of circulating C-peptide/insulin and markers of hyperglycemia [50]. In three prospective cohort studies with follow-up durations of more than 20 years, researchers observed an increased risk of pancreatic cancer among subjects with high postload plasma glucose levels [43,51,52]. In the last of these studies, the risk of pancreatic cancer was 2.2-fold higher in individuals with a postload plasma glucose level greater than 200 mg/dL at baseline than in those with a level of no more than 119 mg/dL [52]. These results indicate that glucose tolerance may precede the onset of pancreatic cancer rather than just be a consequence of this cancer.
Based on these data, long-term type 2 diabetes mellitus likely is an independent risk factor for pancreatic cancer. Additional larger studies are required to further examine the potential confounding effect of smoking and obesity on the association between diabetes and pancreatic cancer.
Mechanisms between Diabetes and Pancreatic Cancer
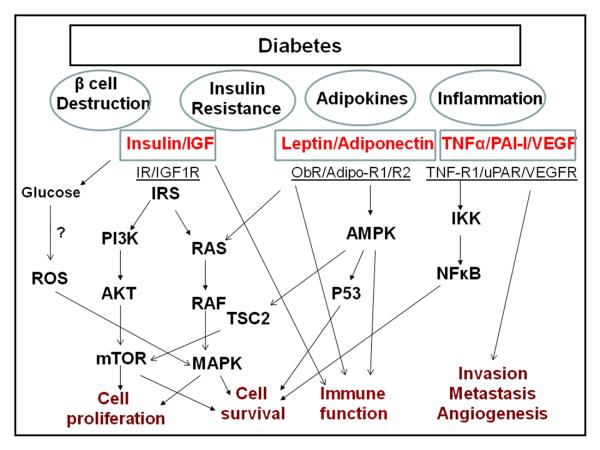
The mechanism of the association between diabetes and pancreatic cancer is elusive but is known to include metabolic, hormonal, and immunological alterations that influence tumor growth (Figure 1). Insulin resistance and compensatory hyperinsulinemia as well as elevated levels of circulating insulin-like growth factors (IGFs) are perhaps the most hypothesized mechanisms underlying the association between type 2 diabetes mellitus and pancreatic cancer. Data from animal studies suggest that islet cell turnover, which is associated with insulin resistance, is critical for pancreatic carcinogenesis. For example, in hamsters, stimulation of islet cell proliferation enhanced pancreatic ductal carcinogenesis [53], and destruction of islet cells by treatment with streptozotocin or alloxan inhibited pancreatic cancer induction [54,55]. Furthermore, treatment with the biguanide metformin inhibited the induction of pancreatic tumors by N-nitrosobis-(2-oxopropyl)amine, a pancreatic carcinogens, and high-fat diets in hamsters by normalizing the rate of islet cell turnover [56].
Fig.1.
Potential mechanisms underlying the associations of diabetes and cancer. AdipoR1/R2, adiponectin receptor 1/2; AMPK, 5′-AMPactivated protein kinase; IGF-1, insulin-like growth factor-1; IGF-1R, insulin-like growth factor-1 receptor; IKK, IκA;B kinase; IR, insulin receptor; IRS-1, insulin receptor substrate-1; MAPK, mitogen-activated-protein-kinase; mTOR, mammalian target of rapamycin; NF-κA;B, nuclear factor-κA;B; ObR, leptin receptor; PAI-1, plasminogen activator inhibitor-1; PI3-K, phosphatidylinositol 3-kinase; ROS, Reactive oxygen species; TNF-α, tumor necrosis factor- α; TNF-R1, tumor necrosis factor-receptor 1; uPA, urokinase-type plasminogen activator; uPAR, urokinase-type plasminogen activator receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Pancreatic β-cell hyperactivity with an increased β-cell mass in the pancreas contributes to insulin oversecretion in response to insulin resistance. The exocrine pancreatic tissue may be chronically exposed to local insulin concentrations that are much higher than the circulating insulin levels seen in hyperinsulinemic patients. Experimental evidence suggests that insulin is a growth-promoting hormone with mitogenic effects. Insulin promotes cell proliferation and increases glucose use [57], both of which are important to tumor development and progression. Furthermore, insulin upregulates the bioavailability of IGFs by reducing hepatic production of IGF-binding proteins [58,59]. The mitogenic and antiapoptotic activities of IGF-1 are more potent than those of insulin and may act as growth stimuli in cells expressing insulin and the IGF-1 receptor (IGF1R). IGF-1 and IGF1R are highly expressed in pancreatic cancer cells [60], and IGF-1–mediated signaling transduction increases proliferation, invasion, and expression of angiogenesis mediators and decreases apoptosis in pancreatic cancer cells [61-63]. IGF1R-mediated initiation of signal transduction activates important intracellular signal pathways, including the Ras/Raf/mitogen-activated protein kinase and phosphoinositide-3 kinase/Akt/mammalian target of rapamycin (mTOR) pathways [64]. Even though epidemiological investigations have not found a significant association between pancreatic cancer risk and prediagnostic plasma levels of IGF-1 and IGF-2 [65,66], investigators have shown that reduced levels of IGF-binding protein 1 are predictive of increased risk of pancreatic cancer [67]. A case-control study indicated a possible association between polymorphic variants of the IGF-1 gene and risk of pancreatic cancer independently of or jointly with diabetes [68].
Abnormal glucose metabolism is a biochemical hallmark of tumor cells, and most tumors have highly effective upregulated, insulin-independent glucose uptake mechanisms. However, evidence implicating a direct role for glucose in promotion of tumor growth is lacking. In animals with diabetes induced by β-cell destruction by alloxan, a study showed that tumor growth was reduced [69], suggesting that hyperglycemia does not increases neoplastic growth, at least in the setting of insulin deficiency. Researchers have suspected that a high dietary glycemic index/load may increase the risk of pancreatic cancer because of the adverse effects of high postprandial glucose levels and resulting insulin demands [70]. Many studies have investigated the associations of carbohydrate and refined sugar intake and glycemic index/load with pancreatic cancer, with inconsistent results [71]. In general, these studies found either no association or weak associations that are not statistically significant. Thus, whether glucose is responsible for the association between hyperglycemia and increased risk of pancreatic cancer or is a surrogate for a causative factor such as insulin resistance remains unclear.
In addition to the direct growth-promoting effect of insulin and IGFs, type 2 diabetes mellitus and/or related obesity may increase the risk of pancreatic cancer by increasing oxidative stress and inflammatory responses. It has been suggested that oxidative stress is an initiating event in the pathogenesis of insulin resistance [72,73]. Also, several epidemiological surveys have reported a correlation between oxidative stress and insulin resistance [74,75]. A causative link between oxidative stress and insulin resistance is further suggested by experimental studies showing that antioxidant supplementation with vitamin E or α-lipoic acid could prevent or reduce insulin resistance [76,77]. In addition, various experimental models of induction of insulin resistance indicated that oxidative stress plays a critical role in the onset of this condition, particularly in animals on high-sucrose or high-fructose diets [78,79]. In these models, hyperglycemia, especially during the postprandial period, was a direct cause of oxidative stress via various mechanisms that may have been related to overproduction of superoxide by the mitochondrial electron-transport chain [80]. Resulting impairment of the cellular redox state both decreased the tyrosine phosphorylation and increased the serine phosphorylation of insulin receptor substrate 1, two events that leads to inactivation of the insulin-signaling pathway [80].
A growing amount of evidence suggests that inflammation plays an important role in pancreatic cancer development [81]. Macronutrient intake and obesity may activate inflammatory signaling pathways [82,83]. Glucose and fat intake may induce inflammation by increasing oxidative stress and the activating transcriptional factors such as nuclear factor-κB, activating protein-1, and early growth response-1 [84-86]. In addition, adipose tissues act as endocrine organs to regulate the release of fatty acids, hormones, and proinflammatory cytokines such as tumor necrosis factor-α, interleukin-6, and resistin [87]. Some adipocytokines are key molecules involved in innate immunity, inflammation, apoptosis, metabolism, and development. Elevated levels of the proinflammatory cytokines promote angiogenesis, tumor progression, and metastasis. Furthermore, altered levels or functions of several molecules previously implicated in obesity and/or diabetes, such as leptin [88], IGF-1 [89], and peroxisome proliferator-activated receptor-γ [90], may contribute to pancreatic cancer development by impairing immune function.
A number of genome-wide association studies have identified unexpected genetic variants that modify the risk of diabetes, and some of the type 2 diabetes mellitus susceptibility loci often tend to be implicated in differentiation and development [91]. Interestingly, one of the pancreatic cancer susceptibility genes identified in genome-wide association studies is NR5A2 (or LRH1) [92], which is a direct target of the pancreatic duodenal homeobox (PDX-1) gene during pancreatic differentiation and development [93]. NR5A2 controls the expression of a number of developmental genes, such as the transcription factors hepatocyte nuclear factor (HNF)-3β, HNF-4α, and HNF-1β. Conversely, expression of NR5A2 is regulated by HNF-3β and HNF-1. PDX-1 is required not only for pancreatic development but also for normal β-cell function and insulin secretion [94]. Mutations of the human HNF-1β gene are linked with maturity-onset diabetes of the young, type 3 [95]. Findings from mouse models with compound heterozygous mutations of PDX-1+/-/HNF-3β+/− and PDX-1+/−/HNF-1β+/− further underscore the importance of PDX-1, HNF-1β, and HNF-3β in controlling glucose tolerance, glucose-stimulated insulin secretion, and islet architecture [96]. Improved understanding of the biological significance of the NR5A2 gene in the development of pancreatic cancer and identification of other genes that modify the risk of diabetes-associated pancreatic cancer would provide further insight into the genetic mechanisms that link diabetes and pancreatic cancer.
Antidiabetic Therapy and Risk of Pancreatic Cancer
Over the past several years, a number of studies of diabetics have shown that treatment with metformin, the most commonly used antidiabetic drug, is associated with lower risk of cancer than is treatment with insulin or insulin secretagogues [97-101]. A meta-analysis of three studies using cancer incidence as the endpoint found a 32% lower overall summary RR for cancer (0.68 [95% CI, 0.52-0.88]) in subjects taking metformin than in those taking other antidiabetic drugs [102]. Regarding pancreatic cancer in particular, a retrospective cohort study of 62,809 patients with diabetes [100] and a case-control study of 973 patients with pancreatic adenocarcinoma and 863 cancer-free controls [103] independently but at nearly the same time demonstrated a very significantly lower risk of pancreatic cancer in metformin users than in insulin or sulfonylurea users. In the retrospective cohort study, the researchers identified 89 pancreatic cancer cases. The hazard ratios in metformin users were 0.20 (95% CI, 0.11-0.36) and 0.22 (95% CI, 0.12-0.38) compared to sulfonylurea users or insulin users, respectively. In the case-control study, the OR for pancreatic cancer was 0.38 (95% CI, 0.21-0.67) in metformin users versus metformin nonusers after adjustment for potential confounders such as smoking status, BMI, family history of cancer, and duration of diabetes. The risk estimates remained statistically significant when the analysis was restricted to patients with diabetes duration of more than 2 years or those who never used insulin. Although the choice of antidiabetic therapy may be related to the severity of the diabetes, which may confound the association between antidiabetic therapy and risk of pancreatic cancer, the validity of these observations is supported by a large amount of experimental evidence of the antitumor activity of metformin [56, 105-108].
In a hamster model of N-nitrosobis-(2-oxopropyl)amine and high-fat diet-induced pancreatic cancer, administration of metformin in drinking water completely prevented tumor development, whereas tumors developed in 50% of the control animals that did not take metformin [56]. Metformin can inhibit proliferation and stimulate apoptosis in tumor cell lines [104] and inhibit the growth of human pancreatic cancer cells xenografted into nude mice [105]. Investigators have also observed the antitumor activity of metformin in various animal models of other common cancers, such as those of the colon [106], breast [107], and lung [108]. More interestingly, a clinical trial conducted in nondiabetic patients with colorectal aberrant crypt foci (ACF) showed that 1 month of metformin use significantly decreased the mean (± standard deviation) number of ACF per patient from 8.78 ± 6.45 before treatment to 5.11 ± 4.99 (P = 0.007), whereas the mean number of ACF did not change significantly in the control group (7.23 ± 6.65 versus 7.56 ± 6.75; P = 0.609) [109]. In patients taking metformin, researchers detected reduced cell proliferation in normal rectal epithelium. These observations suggest that metformin is a promising chemopreventive drug for use against cancer and that use of it may not be limited to patients with diabetes. The challenges that remain are identification of predictive biomarkers for selecting the patients most likely to benefit from such intervention and gaining a clear understanding of the primary mechanism of metformin's antitumor action [110].
Metformin lowers glucose levels mainly by reducing hepatic glucose output [111]. Consequently, metformin decreases rather than increases fasting plasma insulin concentrations and indirectly inhibits the growth-promoting effects of insulin and IGFs. Metformin has been shown to have a cardioprotective effect, causing changes in fat metabolism and production of adipose tissue hormones, particularly leptin [112]. In patients with type 2 diabetes mellitus, metformin-based treatment has been associated with biochemical evidence of improvement of endothelial function, including decreased plasma levels of von Willebrand factor, soluble vascular cell adhesion molecule-1, soluble E-selectin, tissue-type plasminogen activator and inhibitor, and vascular endothelial growth factor [113-116]. Most of these molecules play important roles in thrombosis, fibrosis, and angiogenesis, which may contribute to pancreatic tumor development and progression. However, the main molecular mechanism of metformin's effect on metabolism and growth inhibition are mediated through the liver kinase B1 (LKB1)/5′ AMP-activated protein kinase (AMPK) signaling pathway. In a quiescent cell, most of the energy (in the form of ATP) is generated in the mitochondria through oxidative phosphorylation, including oxidation of fatty acids and amino acids, known as catabolic metabolism. Metformin increases glucose uptake and glycolysis and reduces ATP production in mitochondria, which leads to activation of the LKB1/AMPK signaling pathway.
LKB1 is a tumor suppressor gene, and patients with Peutz-Jeghers syndrome, which is caused by germline mutations of LKB1, have a significantly increased risk of pancreatic cancer [117]. AMPK is a highly conserved sensor of cellular energy status and is activated under conditions with low intracellular ATP levels. AMPK responds to energy stress by suppressing cell growth and biosynthetic processes, in part through its inhibition of the rapamycin-sensitive mTOR complex 1 pathway via tuberous sclerosis 2 protein [118]. mTOR up regulates many energy-consuming cellular processes and has a central role in regulating cell growth by controlling mRNA translation and ribosome biogenesis. AMPK acts as a metabolic checkpoint, coordinating cell growth and energy status to ensure the initiation and maintenance of cell polarity and completion of normal cell division [119]. Also, the LKB1/AMPK pathway regulates p27(kip1) phosphorylation, which permits cells to survive growth factor withdrawal and metabolic stress via autophagy [120]. Studies showed that by altering cellular metabolism, metformin enhanced immune-cell (T-cell) memory over the long term as well as the efficacy of anticancer vaccination [121,122]. Thus, the antitumor activity of metformin results from not only metabolic and hormonal regulation but also cell division and immune functions. Whether metformin or other AMPK agonists can prevent or treat pancreatic cancer deserves further investigation in the laboratory and prospective clinical trials.
Insulin secretagogues such as sulfonylurea, which cause β cells to release insulin, reportedly increase the risk of cancer in patients with type 2 diabetes mellitus [98,100,123]. However, most of the studies included very few cases of cancer; therefore, no specific cancer sites were implicated. Two studies that showed a protective effect of metformin on pancreatic cancer also demonstrated an increased risk of this cancer associated with use of sulfonylurea [100,103]. Because type 2 diabetes mellitus is a progressive disease, few patients with it receive monotherapy over the entire disease course, so most patients receive combined therapy with different medications. Therefore, determining whether the observed risk associations reflect increased risk in sulfonylurea users or decreased risk in comparative groups, such as metformin users, is difficult.
Because of the tumor-promoting effect of an elevated level of insulin and resulting IGF-1, whether the use of insulin in diabetic patients increases the risk of cancer has garnered a great deal of attention. Previous studies have reviewed and debated this issue [124,125]. In general, evidence that insulin use increases the risk of cancer is insufficient [126]. Although insulin use has been associated with an increased risk of pancreatic cancer, the risk was usually restricted to patients who took insulin for a short time (<3 years) [22,23,25,26,48]. In a pooled analysis of data from three large case-control studies [48], long-term insulin use (>10 years) was associated with a reduced risk of pancreatic cancer, whereas the risk in those who used insulin for 3-10 years was 20% higher than that in diabetics who did not use insulin. In another study, among patients with pancreatic cancer, more than 70% of insulin users began receiving it at least 2 years before their cancer diagnoses [127]. These cases were either pancreatic cancer caused by diabetes or long-term diabetes aggravated by the cancer. In either type of case, the association between short-term insulin use and pancreatic cancer is most likely a result of reverse causality. Of four published case-control studies that examined the duration of insulin use and risk of pancreatic cancer, three studies demonstrated no association or a reduced risk in those who used insulin for more than 5 years [22,23,26]. No experimental evidence has shown that improved glycemic control with insulin use actually reduces the risk of pancreatic cancer. Most analyses of pancreatic cancer risk in diabetics have had limited numbers of insulin users; larger sample sizes are required to fully assess any possible effect of insulin use on the risk of pancreatic cancer.
Pancreatic Cancer-Associated Diabetes and Implication in Early Detection of this Cancer
It has been reported that the prevalence of diabetes and impaired glucose tolerance in pancreatic cancer cases is as high as 80%. In the majority of cases, diabetes associated with pancreatic cancer is diagnosed fewer than 2 years prior to the cancer diagnosis or during the cancer course. Among patients with early-stage pancreatic cancer, diabetes has developed more often in patients with carcinoma of the head of the pancreas than in those with carcinoma of the body and/or tail of the organ [5]. However, why diabetes develops in patients with pancreatic cancer remains unclear. One hypothesis is that cancer-causing agents such as tobacco carcinogens and cancer cell products cause islet cell destruction, resulting in β-cell dysfunction and diabetes. Reduced insulin release in response to glucose in animals with chemically induced pancreatic tumors [128] and in patients with pancreatic cancer [129] have been observed. However, clinical studies have shown that the majority of patients with pancreatic cancer have normal β-cell function [130], and diabetes in pancreatic cancer cases is characterized by peripheral insulin resistance. Another hypothesis is that diabetes is caused by biliary obstruction and distal pancreatitis associated with a tumor [5]. An early study showed a higher frequency of elevated bilirubin levels in patients with pancreatic cancer who had abnormal glucose metabolism (66%) than in patients with no evidence of disturbed glucose metabolism (51%) [131]. The finding that insulin resistance and diabetes improved markedly 3 weeks after tumor resection in patients with pancreatic cancer suggests a causal role for tumors in the development of diabetes in patients with pancreatic cancer [132]. Interestingly, pancreatic cancer cells reportedly produced unidentified factors that were able to induce hyperglycemia in severe combined immunodeficiency mice [133] and altered glucose metabolism in cultured rat hepatocytes [134]. Some of the putative “diabetogenic factors” have been identified as the S-100A8 N-terminal peptide [135,136]. Another molecule that has been suggested to be a diabetogenic factor is the islet amyloid peptide (IAPP). IAPP is a hormonal factor secreted from pancreatic β cells that reduces insulin sensitivity in vivo and glycogen synthesis in vitro [137]. An increased level of IAPP was found in the plasma of patients with pancreatic cancer and diabetes and the level was significantly reduced after tumor resection, supporting a role for IAPP in the development of insulin resistance and diabetes in patients with pancreatic cancer [138]. However, elevated plasma level of IAPP has also been found in chronic pancreatitis, other gastrointestinal malignancies and biliary obstruction thus is not specific for pancreatic cancer [139,140]. A recent study has shown that pancreas tissues from patients with diabetes and tumor tissues form patients with pancreatic cancer had reduced number of IAPP-expressing cells and β cells while the normal adjacent tissues from patients with pancreatic cancer had increased number of IAPP-expressing cells and β cells but reduced number of α cells [141]. These findings did not support the hypothesis that undiagnosed diabetes or impaired glucose tolerance is the main cause of the pancreatic cancer-associated diabetes. The difference in the expression of IAPP between tumor and normal adjacent tissues also suggests the role of cancer cells in the expression of IAPP.
Findings from a retrospective cohort study of 2122 diabetic patients suggested that pancreatic cancer developed within 3 years after the diagnosis of diabetes in 1% of the patients who were at least 50 years old [142]. In a later case-control study of pancreatic cancer including detailed fasting blood glucose level data up to 60 months before cancer diagnosis in cases or recruitment to the study in controls, pancreatic cancer-induced hyperglycemia developed up to 24-36 months prior to the cancer diagnosis [143]. These observations suggest that diabetes is a biomarker of early-stage pancreatic cancer and that identifying patients with cancer-associated diabetes at the onset of their diabetes would offer the opportunity for early detection of pancreatic cancer. However, screening for exocrine pancreatic cancer in patients with newly diagnosed diabetes may not be practical, as only 2% of them have tumors, and computed tomographic and endoscopic ultrasonographic scanning of the pancreas as screening procedures are neither cost-effective nor adequately sensitive or specific. Defining biomarkers that may help identify new-onset diabetes in patients with the greatest risk of pancreatic cancer for diagnostic clinical screening is an urgent need. Carbohydrate antigen 19-9 is the most commonly used biomarker for diagnosis of and monitoring treatment response to pancreatic cancer. However, as a screening marker in asymptomatic patients, carbohydrate antigen 19-9 had a predictive value of less than 1%, because its level was highly associated with tumor size [144,145]. A gene expression profiling study using peripheral lymphocytes suggested that expression of vanin 1 and matrix metalloproteinase 9 together may be useful in discriminating pancreatic cancer-associated diabetes from type 2 diabetes mellitus [146]. Further research in this area is important and promising for early detection of pancreatic cancer.
Diabetes and Clinical Outcome of Pancreatic Cancer
Increasing evidence suggests that patients with certain types of cancer, such as colon, endometrial, and breast cancer and leukemia, who also have diabetes or impaired glucose tolerance are at increased risk for cancer recurrence, cancer-related death, and death due to any cause [147-151]. These studies also indicated that hormonal or metabolic abnormalities, such as hyperinsulinemia and hyperglycemia, affect tumor biology at multiple stages, including malignant transformation, growth, and metastasis. For certain malignancies, metabolic abnormalities associated with diabetes and impaired glucose tolerance apparently have an adverse effect on the response of these malignancies to treatment [149,150,152]. Despite the high prevalence of diabetes in patients with pancreatic cancer, the available information on how diabetes affects these patients' treatment and clinical outcomes is limited. A few studies have examined the associations between diabetes and survival of pancreatic cancer, but their findings have been inconsistent. Some studies found that diabetes did not have a significant effect on the overall survival time [149,153,154] or on the mortality of pancreatic cancer [45], whereas others found that diabetes was associated with significantly reduced survival durations [5,155-157]. These inconsistent findings may be partially explained by the different patient populations studied, as the negative effect of diabetes on survival occurred mostly in patients with resected pancreatic tumors, whereas patients with late-stage disease had no effect of diabetes on survival. In addition, few of these studies considered the potential confounding effect of obesity, which has exhibited a significant impact on survival in patients with pancreatic cancer [158,159]. Because most of the current therapeutic approaches for cancer treatment that focus on inhibition of cell proliferation and death signaling pathways do not significantly impact the survival of pancreatic cancer, new therapeutic regimens targeting energy metabolism or inflammation via inhibition of the insulin/IGF axis signaling pathway and modulation of lipid metabolism may hold great promise in the treatment of pancreatic cancer, especially that associated with diabetes and obesity [160].
Conclusion
The relationship between diabetes and pancreatic cancer is complex. The observed causal relationship between long-term type 2 diabetes mellitus and pancreatic cancer and the protective effect of the antidiabetic drug metformin against pancreatic cancer offer opportunities for the prevention and intervention of this cancer. Increased understand of the mechanisms of the relationship between diabetes and pancreatic cancer would provide new research avenues for the development of novel preventive and therapeutic strategies for this deadly disease.
Acknowledgments
This research is supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672, NIH grant RO1 CA098380 and National Institute of Environmental Sciences Center grant P30 ES07784.
Abbreviations
- ACF
aberrant crypt foci
- AMPK
5′ AMP-activated protein kinase
- BMI
body mass index
- CI
confidence interval
- IAPP
islet amyloid peptide
- IGF
insulin-like growth factor
- LKB1
liver kinase B1
- mTOR
mammalian target of rapamycin
- OR
odds ratio
- RR
relative risk
REFERENCES
- 1.Noy A, Bilezikian JP. Clinical review 63: Diabetes and pancreatic cancer: clues to the early diagnosis of pancreatic malignancy. J Clin Endocrinol Metab. 1994;79:1223–31. doi: 10.1210/jcem.79.5.7962312. [DOI] [PubMed] [Google Scholar]
- 2.Gullo L, Pezzilli R. Diabetes and pancreatic cancer. Pancreas. 2004;28:451. doi: 10.1097/00006676-200405000-00018. author reply 451-2. [DOI] [PubMed] [Google Scholar]
- 3.Fisher WE. Diabetes: risk factor for the development of pancreatic cancer or manifestation of the disease? World J Surg. 2001;25:503–8. doi: 10.1007/s002680020344. [DOI] [PubMed] [Google Scholar]
- 4.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnqvist HJ, Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–7. [PubMed] [Google Scholar]
- 5.Wakasugi H, Funakoshi A, Iguchi H. Clinical observations of pancreatic diabetes caused by pancreatic carcinoma, and survival period. Int J Clin Oncol. 2001;6:50–4. doi: 10.1007/pl00012080. [DOI] [PubMed] [Google Scholar]
- 6.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–7. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekoe JM, Ghadirian P, Simard A, Baillargeon J, Perret C. Diabetes mellitus and pancreatic cancer: a case-control study in greater Montreal, Quebec, Canada. Rev Epidemiol Sante Publique. 1992;40:447–53. [PubMed] [Google Scholar]
- 8.Wynder EL, Mabuchi K, Maruchi N, Fortner JG. A case control study of cancer of the pancreas. Cancer. 1973;31:641–8. doi: 10.1002/1097-0142(197303)31:3<641::aid-cncr2820310323>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Lin RS, Kessler II. A multifactorial model for pancreatic cancer in man. Epidemiologic evidence. JAMA. 1981;245:147–52. [PubMed] [Google Scholar]
- 10.Gold EB, Gordis L, Diener MD, Seltser R, Boitnott JK, Bynum TE, Hutcheon DF. Diet and other risk factors for cancer of the pancreas. Cancer. 1985;55:460–7. doi: 10.1002/1097-0142(19850115)55:2<460::aid-cncr2820550229>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.O'Mara BA, Byers T, Schoenfeld E. Diabetes mellitus and cancer risk: a multisite case-control study. J Chronic Dis. 1985;38:435–41. doi: 10.1016/0021-9681(85)90139-0. [DOI] [PubMed] [Google Scholar]
- 12.Norell S, Ahlbom A, Erwald R, Jacobson G, Lindberg-Navier I, Olin R, Wiechel KL. Diabetes, gall stone disease, and pancreatic cancer. Br J Cancer. 1986;54:377–8. doi: 10.1038/bjc.1986.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuzick J, Babiker AG. Pancreatic cancer, alcohol, diabetes mellitus and gall-bladder disease. Int J Cancer. 1989;43:415–21. doi: 10.1002/ijc.2910430312. [DOI] [PubMed] [Google Scholar]
- 14.Farrow DC, Davis S. Risk of pancreatic cancer in relation to medical history and the use of tobacco, alcohol and coffee. Int J Cancer. 1990;45:816–20. doi: 10.1002/ijc.2910450504. [DOI] [PubMed] [Google Scholar]
- 15.Jain M, Howe GR, St Louis P, Miller AB. Coffee and alcohol as determinants of risk of pancreas cancer: a case-control study from Toronto. Int J Cancer. 1991;47:384–9. doi: 10.1002/ijc.2910470313. [DOI] [PubMed] [Google Scholar]
- 16.Kalapothaki V, Tzonou A, Hsieh CC, Toupadaki N, Karakatsani A, Trichopoulos D. Tobacco, ethanol, coffee, pancreatitis, diabetes mellitus, and cholelithiasis as risk factors for pancreatic carcinoma. Cancer Causes Control. 1993;4:375–82. doi: 10.1007/BF00051341. [DOI] [PubMed] [Google Scholar]
- 17.La Vecchia C, Negri E, Franceschi S, D'Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70:950–3. doi: 10.1038/bjc.1994.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331:81–4. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 19.Bueno de Mesquita HB, Maisonneuve P, Moerman CJ, Walker AM. Aspects of medical history and exocrine carcinoma of the pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1992;52:17–23. doi: 10.1002/ijc.2910520105. [DOI] [PubMed] [Google Scholar]
- 20.Lee CT, Chang FY, Lee SD. Risk factors for pancreatic cancer in orientals. J Gastroenterol Hepatol. 1996;11:491–5. doi: 10.1111/j.1440-1746.1996.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 21.Frye JN, Inder WJ, Dobbs BR, Frizelle FA. Pancreatic cancer and diabetes: is there a relationship? A case-controlled study. Aust N Z J Surg. 2000;70:722–4. doi: 10.1046/j.1440-1622.2000.01940.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area, California. Cancer Epidemiol Biomarkers Prev. 2006;15:1458–63. doi: 10.1158/1055-9965.EPI-06-0188. [DOI] [PubMed] [Google Scholar]
- 23.Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, Khan R, Chou TH, Lenzi R, Jiao L, Li D. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102:2696–707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMahon B, Yen S, Trichopoulos D, Warren K, Nardi G. Coffee and cancer of the pancreas. N Engl J Med. 1981;304:630–3. doi: 10.1056/NEJM198103123041102. [DOI] [PubMed] [Google Scholar]
- 25.Bonelli L, Aste H, Bovo P, Cavallini G, Felder M, Gusmaroli R, Morandini E, Ravelli P, Briglia R, Lombardo L, De Micheli A, Pugliese V. Exocrine pancreatic cancer, cigarette smoking, and diabetes mellitus: a case-control study in northern Italy. Pancreas. 2003;27:143–9. doi: 10.1097/00006676-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, Schwartz AG, Brown LM, Greenberg RS, Schoenberg JB, Pottern LM, Hoover RN, Fraumeni JF., Jr. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80:1830–7. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler II. Cancer mortality among diabetics. J Natl Cancer Inst. 1970;44:673–86. [PubMed] [Google Scholar]
- 28.Ragozzino M, Melton LJ, 3rd, Chu CP, Palumbo PJ. Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis. 1982;35:13–9. doi: 10.1016/0021-9681(82)90025-x. [DOI] [PubMed] [Google Scholar]
- 29.Whittemore AS, Paffenbarger RS, Jr., Anderson K, Halpern J. Early precursors of pancreatic cancer in college men. J Chronic Dis. 1983;36:251–6. doi: 10.1016/0021-9681(83)90059-0. [DOI] [PubMed] [Google Scholar]
- 30.Mills PK, Beeson WL, Abbey DE, Fraser GE, Phillips RL. Dietary habits and past medical history as related to fatal pancreas cancer risk among Adventists. Cancer. 1988;61:2578–85. doi: 10.1002/1097-0142(19880615)61:12<2578::aid-cncr2820611232>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Hiatt RA, Klatsky AL, Armstrong MA. Pancreatic cancer, blood glucose and beverage consumption. Int J Cancer. 1988;41:794–7. doi: 10.1002/ijc.2910410603. [DOI] [PubMed] [Google Scholar]
- 32.Balkau B, Barrett-Connor E, Eschwege E, Richard JL, Claude JR, Ducimetiere P. Diabetes and pancreatic carcinoma. Diabete Metab. 1993;19:458–62. [PubMed] [Google Scholar]
- 33.Shibata A, Mack TM, Paganini-Hill A, Ross RK, Henderson BE. A prospective study of pancreatic cancer in the elderly. Int J Cancer. 1994;58:46–9. doi: 10.1002/ijc.2910580109. [DOI] [PubMed] [Google Scholar]
- 34.Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, Persson I. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2:307–14. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 35.Friedman GD, van den Eeden SK. Risk factors for pancreatic cancer: an exploratory study. Int J Epidemiol. 1993;22:30–7. doi: 10.1093/ije/22.1.30. [DOI] [PubMed] [Google Scholar]
- 36.Chow WH, Gridley G, Nyren O, Linet MS, Ekbom A, Fraumeni JF, Jr., Adami HO. Risk of pancreatic cancer following diabetes mellitus: a nationwide cohort study in Sweden. J Natl Cancer Inst. 1995;87:930–1. doi: 10.1093/jnci/87.12.930. [DOI] [PubMed] [Google Scholar]
- 37.Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–5. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 38.Lund Nilsen TI, Johnsen R, Vatten LJ. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br J Cancer. 2000;82:1358–63. doi: 10.1054/bjoc.1999.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland) Cancer Causes Control. 2002;13:417–26. doi: 10.1023/a:1015729615148. [DOI] [PubMed] [Google Scholar]
- 40.Lin Y, Tamakoshi A, Kawamura T, Inaba Y, Kikuchi S, Motohashi Y, Kurosawa M, Ohno Y. Risk of pancreatic cancer in relation to alcohol drinking, coffee consumption and medical history: findings from the Japan collaborative cohort study for evaluation of cancer risk. Int J Cancer. 2002;99:742–6. doi: 10.1002/ijc.10402. [DOI] [PubMed] [Google Scholar]
- 41.Inoue M, Tajima K, Takezaki T, Hamajima N, Hirose K, Ito H, Tominaga S. Epidemiology of pancreatic cancer in Japan: a nested case-control study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC) Int J Epidemiol. 2003;32:257–62. doi: 10.1093/ije/dyg062. [DOI] [PubMed] [Google Scholar]
- 42.Rulyak SJ, Lowenfels AB, Maisonneuve P, Brentnall TA. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292–9. doi: 10.1016/s0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- 43.Batty GD, Shipley MJ, Marmot M, Smith GD. Diabetes status and post-load plasma glucose concentration in relation to site-specific cancer mortality: findings from the original Whitehall study. Cancer Causes Control. 2004;15:873–81. doi: 10.1007/s10552-004-1050-z. [DOI] [PubMed] [Google Scholar]
- 44.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 45.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–64. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605–9. [PubMed] [Google Scholar]
- 47.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, Tang H, Hassan M, Holly EA, Bracci PM, Silverman DT. Diabetes and Risk of Pancreatic Cancer: A Pooled Analysis of Three Large Case-Control Studies. Cancer Causes & Control. doi: 10.1007/s10552-010-9686-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, Albanes D. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–8. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 50.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 51.Smith GD, Egger M, Shipley MJ, Marmot MG. Post-challenge glucose concentration, impaired glucose tolerance, diabetes, and cancer mortality in men. Am J Epidemiol. 1992;136:1110–4. doi: 10.1093/oxfordjournals.aje.a116576. [DOI] [PubMed] [Google Scholar]
- 52.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–8. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 53.Pour PM, Kazakoff K. Stimulation of islet cell proliferation enhances pancreatic ductal carcinogenesis in the hamster model. Am J Pathol. 1996;149:1017–25. [PMC free article] [PubMed] [Google Scholar]
- 54.Pour PM, Donnelly K, Stepan K. Modification of pancreatic carcinogenesis in the hamster model. 3. Inhibitory effect of alloxan. Am J Pathol. 1983;110:310–4. [PMC free article] [PubMed] [Google Scholar]
- 55.Pour PM, Patil K. Modification of pancreatic carcinogenesis in the hamster model. X. Effect of streptozotocin. J Natl Cancer Inst. 1983;71:1059–65. [PubMed] [Google Scholar]
- 56.Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, Adrian TE, Pour PM. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120:1263–70. doi: 10.1053/gast.2001.23258. [see comment] [DOI] [PubMed] [Google Scholar]
- 57.Ding XZ, Fehsenfeld DM, Murphy LO, Permert J, Adrian TE. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas. 2000;21:310–20. doi: 10.1097/00006676-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Ooi GT, Tseng LY, Tran MQ, Rechler MM. Insulin rapidly decreases insulin-like growth factor-binding protein-1 gene transcription in streptozotocin-diabetic rats. Mol Endocrinol. 1992;6:2219–28. doi: 10.1210/mend.6.12.1283442. [DOI] [PubMed] [Google Scholar]
- 59.Powell DR, Suwanichkul A, Cubbage ML, DePaolis LA, Snuggs MB, Lee PD. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem. 1991;266:18868–76. [PubMed] [Google Scholar]
- 60.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Research. 1995;55:2007–11. [PubMed] [Google Scholar]
- 61.Ohmura E, Okada M, Onoda N, Kamiya Y, Murakami H, Tsushima T, Shizume K. Insulin-like growth factor I and transforming growth factor alpha as autocrine growth factors in human pancreatic cancer cell growth. Cancer Res. 1990;50:103–7. [PubMed] [Google Scholar]
- 62.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, Evans DB, Semenza GL, Ellis LM. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. American Journal of Pathology. 2003;163:1001–11. doi: 10.1016/s0002-9440(10)63460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng H, Datta K, Neid M, Li J, Parangi S, Mukhopadhyay D. Requirement of different signaling pathways mediated by insulin-like growth factor-I receptor for proliferation, invasion, and VPF/VEGF expression in a pancreatic carcinoma cell line. Biochemical & Biophysical Research Communications. 2003;302:46–55. doi: 10.1016/s0006-291x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 64.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 65.Stolzenberg-Solomon RZ, Limburg P, Pollak M, Taylor PR, Virtamo J, Albanes D. Insulin-like growth factor (IGF)-1, IGF-binding protein-3, and pancreatic cancer in male smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:438–44. [PubMed] [Google Scholar]
- 66.Wolpin BM, Michaud DS, Giovannucci EL, Schernhammer ES, Stampfer MJ, Manson JE, Cochrane BB, Rohan TE, Ma J, Pollak MN, Fuchs CS. Circulating insulin-like growth factor axis and the risk of pancreatic cancer in four prospective cohorts. Br J Cancer. 2007;97:98–104. doi: 10.1038/sj.bjc.6603826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolpin BM, Michaud DS, Giovannucci EL, Schernhammer ES, Stampfer MJ, Manson JE, Cochrane BB, Rohan TE, Ma J, Pollak MN, Fuchs CS. Circulating insulin-like growth factor binding protein-1 and the risk of pancreatic cancer. Cancer Res. 2007;67:7923–8. doi: 10.1158/0008-5472.CAN-07-0373. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki H, Li Y, Dong X, Hassan MM, Abbruzzese JL, Li D. Effect of insulin-like growth factor gene polymorphisms alone or in interaction with diabetes on the risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3467–73. doi: 10.1158/1055-9965.EPI-08-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heuson JC, Legros N. Influence of insulin deprivation on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats subjected to alloxan diabetes and food restriction. Cancer Res. 1972;32:226–32. [PubMed] [Google Scholar]
- 70.Hine RJ, Srivastava S, Milner JA, Ross SA. Nutritional links to plausible mechanisms underlying pancreatic cancer: a conference report. Pancreas. 2003;27:356–66. doi: 10.1097/00006676-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 71.Mulholland HG, Murray LJ, Cardwell CR, Cantwell MM. Glycemic index, glycemic load, and risk of digestive tract neoplasms: a systematic review and meta-analysis. Am J Clin Nutr. 2009;89:568–76. doi: 10.3945/ajcn.2008.26823. [DOI] [PubMed] [Google Scholar]
- 72.Ogihara T, Asano T, Katagiri H, Sakoda H, Anai M, Shojima N, Ono H, Fujishiro M, Kushiyama A, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Gotoh Y, Komuro I, Fujita T. Oxidative stress induces insulin resistance by activating the nuclear factor-kappa B pathway and disrupting normal subcellular distribution of phosphatidylinositol 3-kinase. Diabetologia. 2004;47:794–805. doi: 10.1007/s00125-004-1391-x. [DOI] [PubMed] [Google Scholar]
- 73.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 74.Gopaul NK, Manraj MD, Hebe A, Lee Kwai Yan S, Johnston A, Carrier MJ, Anggard EE. Oxidative stress could precede endothelial dysfunction and insulin resistance in Indian Mauritians with impaired glucose metabolism. Diabetologia. 2001;44:706–12. doi: 10.1007/s001250051679. [DOI] [PubMed] [Google Scholar]
- 75.Manning PJ, Sutherland WH, Walker RJ, Williams SM, De Jong SA, Ryalls AR, Berry EA. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care. 2004;27:2166–71. doi: 10.2337/diacare.27.9.2166. [DOI] [PubMed] [Google Scholar]
- 76.Midaoui AE, Elimadi A, Wu L, Haddad PS, de Champlain J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am J Hypertens. 2003;16:173–9. doi: 10.1016/s0895-7061(02)03253-3. [DOI] [PubMed] [Google Scholar]
- 77.Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, Stas S, Kumar P, Sha Q, Ferrario CM, Sowers JR. Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab. 2005;288:E353–9. doi: 10.1152/ajpendo.00402.2004. [DOI] [PubMed] [Google Scholar]
- 78.Busserolles J, Rock E, Gueux E, Mazur A, Grolier P, Rayssiguier Y. Short-term consumption of a high-sucrose diet has a pro-oxidant effect in rats. Br J Nutr. 2002;87:337–42. doi: 10.1079/BJNBJN2002524. [DOI] [PubMed] [Google Scholar]
- 79.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 80.Tirosh A, Potashnik R, Bashan N, Rudich A. Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. A putative cellular mechanism for impaired protein kinase B activation and GLUT4 translocation. J Biol Chem. 1999;274:10595–602. doi: 10.1074/jbc.274.15.10595. [DOI] [PubMed] [Google Scholar]
- 81.Greer JB, Whitcomb DC, Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Current Opinion in Pharmacology. 2009;9:411–8. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 82.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 83.Gallagher EJ, LeRoith D, Gallagher EJ, LeRoith D. Insulin, insulin resistance, obesity, and cancer. Current Diabetes Reports. 2010;10:93–100. doi: 10.1007/s11892-010-0101-y. [DOI] [PubMed] [Google Scholar]
- 84.Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr. 2004;80:51–7. doi: 10.1093/ajcn/80.1.51. [DOI] [PubMed] [Google Scholar]
- 85.Dhindsa S, Tripathy D, Mohanty P, Ghanim H, Syed T, Aljada A, Dandona P. Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-kappaB in mononuclear cells. Metabolism. 2004;53:330–4. doi: 10.1016/j.metabol.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 86.Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr. 2002;75:767–72. doi: 10.1093/ajcn/75.4.767. [DOI] [PubMed] [Google Scholar]
- 87.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–78. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 88.Lago R, Gómez R, Lago F, Gómez-Reino J, Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cellular Immunology. 2008;252:139–45. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 89.Smith TJ, Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacological Reviews. 2010;62:199–236. doi: 10.1124/pr.109.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanisms. J Immunol. 2007;178:4129–35. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- 91.Duffy DL, Duffy DL. Genetic determinants of diabetes are similarly associated with other immune-mediated diseases. Current Opinion in Allergy & Clinical Immunology. 2007;7:468–74. doi: 10.1097/ACI.0b013e3282f1dc99. [DOI] [PubMed] [Google Scholar]
- 92.Petersen GM, Amundadottir L, Fuchs CS, Kraft P, Stolzenberg-Solomon RZ, Jacobs KB, Arslan AA, Bueno-de-Mesquita HB, Gallinger S, Gross M, Helzlsouer K, Holly EA, Jacobs EJ, Klein AP, LaCroix A, Li D, Mandelson MT, Olson SH, Risch HA, Zheng W, Albanes D, Bamlet WR, Berg CD, Boutron-Ruault MC, Buring JE, Bracci PM, Canzian F, Clipp S, Cotterchio M, de Andrade M, Duell EJ, Gaziano JM, Giovannucci EL, Goggins M, Hallmans G, Hankinson SE, Hassan M, Howard B, Hunter DJ, Hutchinson A, Jenab M, Kaaks R, Kooperberg C, Krogh V, Kurtz RC, Lynch SM, McWilliams RR, Mendelsohn JB, Michaud DS, Parikh H, Patel AV, Peeters PH, Rajkovic A, Riboli E, Rodriguez L, Seminara D, Shu XO, Thomas G, Tjonneland A, Tobias GS, Trichopoulos D, Van Den Eeden SK, Virtamo J, Wactawski-Wende J, Wang Z, Wolpin BM, Yu H, Yu K, Zeleniuch-Jacquotte A, Fraumeni JF, Jr., Hoover RN, Hartge P, Chanock SJ. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–8. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Annicotte JS, Fayard E, Swift GH, Selander L, Edlund H, Tanaka T, Kodama T, Schoonjans K, Auwerx J. Pancreatic-duodenal homeobox 1 regulates expression of liver receptor homolog 1 during pancreas development. Molecular & Cellular Biology. 2003;23:6713–24. doi: 10.1128/MCB.23.19.6713-6724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277:11225–32. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 95.Glucksmann MA, Lehto M, Tayber O, Scotti S, Berkemeier L, Pulido JC, Wu Y, Nir WJ, Fang L, Markel P, Munnelly KD, Goranson J, Orho M, Young BM, Whitacre JL, McMenimen C, Wantman M, Tuomi T, Warram J, Forsblom CM, Carlsson M, Rosenzweig J, Kennedy G, Duyk GM, Thomas JD, et al. Novel mutations and a mutational hotspot in the MODY3 gene. Diabetes. 1997;46:1081–6. doi: 10.2337/diab.46.6.1081. [DOI] [PubMed] [Google Scholar]
- 96.Shih DQ, Heimesaat M, Kuwajima S, Stein R, Wright CV, Stoffel M. Profound defects in pancreatic beta-cell function in mice with combined heterozygous mutations in Pdx-1, Hnf-1alpha, and Hnf-3beta. Proc Natl Acad Sci U S A. 2002;99:3818–23. doi: 10.1073/pnas.062605899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [see comment] [DOI] [PubMed] [Google Scholar]
- 98.Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E, Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetologica. 2009;46:279–84. doi: 10.1007/s00592-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 99.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JMM. New Users of Metformin Are at Low Risk of Incident Cancer. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 101.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ, Landman GWD, Kleefstra N, van Hateren KJJ, Groenier KH, Gans ROB, Bilo HJG. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–6. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and Cancer Risk in Diabetic Patients: A Systematic Review and Meta-analysis. Cancer Prev Res (Phila) 3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 103.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, Thor AD. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–15. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 105.Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–45. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, Suzuki K, Nozaki Y, Yoneda K, Fujita K, Yoneda M, Inamori M, Tomatsu A, Chihara T, Shimpo K, Nakagama H, Nakajima A. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49:662–71. doi: 10.1002/mc.20637. [DOI] [PubMed] [Google Scholar]
- 107.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Experimental Gerontology. 2005;40:685–93. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin Prevents Tobacco Carcinogen-Induced Lung Tumorigenesis. Cancer Prev Res. 2010;3:1066–76. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, Koide T, Tokoro C, Abe Y, Inamori M, Nakagama H, Nakajima A. Metformin Suppresses Colorectal Aberrant Crypt Foci in a Short-term Clinical Trial. Cancer Prev Res. 2010;3:1077–83. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 110.Pollak M. Metformin and Other Biguanides in Oncology: Advancing the Research Agenda. Cancer Prev Res (Phila Pa) 2010;3:1060–65. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 112.Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs. 2003;63:1879–94. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 113.Ersoy C, Kiyici S, Budak F, Oral B, Guclu M, Duran C, Selimoglu H, Erturk E, Tuncel E, Imamoglu S. The effect of metformin treatment on VEGF and PAI-1 levels in obese type 2 diabetic patients. Diabetes Res Clin Pract. 2008;81:56–60. doi: 10.1016/j.diabres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 114.De Jager J, Kooy A, Lehert P, Bets D, Wulffele MG, Teerlink T, Scheffer PG, Schalkwijk CG, Donker AJ, Stehouwer CD. Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2005;257:100–9. doi: 10.1111/j.1365-2796.2004.01420.x. [DOI] [PubMed] [Google Scholar]
- 115.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–7. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 116.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37:1344–50. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 117.Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–53. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 118.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Williams T, Brenman JE. LKB1 and AMPK in cell polarity and division. Trends in Cell Biology. 2008;18:193–8. doi: 10.1016/j.tcb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 120.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–24. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 121.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prlic M, Bevan MJ. Immunology: A metabolic switch to memory. Nature. 2009;460:41–2. doi: 10.1038/460041a. [DOI] [PubMed] [Google Scholar]
- 123.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD, Evans JMM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gerstein HC. Does insulin therapy promote, reduce, or have a neutral effect on cancers? JAMA. 2010;303:446–7. doi: 10.1001/jama.2010.60. [DOI] [PubMed] [Google Scholar]
- 125.Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 126.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. CA: a Cancer Journal for Clinicians. 2010;60:207–21. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 127.Li D, Yeung SJ, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic Therapy and Risk of Pancreatic Cancer. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahren B, Andren-Sandberg A. Glucose tolerance and insulin secretion in experimental pancreatic cancer in the Syrian hamster. Res Exp Med (Berl) 1993;193:21–6. doi: 10.1007/BF02576207. [DOI] [PubMed] [Google Scholar]
- 129.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–93. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 130.Fogar P, Basso D, Panozzo MP, Del Favero G, Briani G, Fabris C, D'Angeli F, Meggiato T, Ferrara C, Plebani M. C-peptide pattern in patients with pancreatic cancer. Anticancer Research. 1993;13:2577–80. [PubMed] [Google Scholar]
- 131.Murphy R, Smith FH. Abnormal carbohydrate metabolism in pancreatic carcinoma. Med Clin North Am. 1963;47:397–405. doi: 10.1016/s0025-7125(16)33598-2. [DOI] [PubMed] [Google Scholar]
- 132.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–50. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 133.Basso D, Brigato L, Veronesi A, Panozzo MP, Amadori A, Plebani M. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Research. 1995;15:2585–8. [PubMed] [Google Scholar]
- 134.Basso D, Valerio A, Brigato L, Panozzo MP, Miola M, Lucca T, Ujka F, Zaninotto M, Avogaro A, Plebani M. An unidentified pancreatic cancer cell product alters some intracellular pathways of glucose metabolism in isolated rat hepatocytes. Pancreas. 1997;15:132–8. doi: 10.1097/00006676-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 135.Basso D, Valerio A, Seraglia R, Mazza S, Piva MG, Greco E, Fogar P, Gallo N, Pedrazzoli S, Tiengo A, Plebani M. Putative pancreatic cancer-associated diabetogenic factor: 2030 MW peptide. Pancreas. 2002;24:8–14. doi: 10.1097/00006676-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 136.Basso D, Greco E, Fogar P, Pucci P, Flagiello A, Baldo G, Giunco S, Valerio A, Navaglia F, Zambon CF, Falda A, Pedrazzoli S, Plebani M. Pancreatic cancer-derived S-100A8 N-terminal peptide: a diabetes cause? Clin Chim Acta. 2006;372:120–8. doi: 10.1016/j.cca.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 137.Wang F, Adrian TE, Westermark G, Gasslander T, Permert J. Dissociated insulin and islet amyloid polypeptide secretion from isolated rat pancreatic islets cocultured with human pancreatic adenocarcinoma cells. Pancreas. 1999;18:403–9. doi: 10.1097/00006676-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 138.Permert J, Larsson J, Westermark GT, Herrington MK, Christmanson L, Pour PM, Westermark P, Adrian TE. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med. 1994;330:313–8. doi: 10.1056/NEJM199402033300503. [DOI] [PubMed] [Google Scholar]
- 139.Brand RE, Ding XZ, Young CM, Adrian TE, Brand RE, Ding X-Z, Young CM, Adrian TE. The specificity of amylin for the diagnosis of pancreatic adenocarcinoma. International Journal of Gastrointestinal Cancer. 2002;31:123–8. doi: 10.1385/IJGC:31:1-3:123. [DOI] [PubMed] [Google Scholar]
- 140.Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–5. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 141.Saruc M, Iki K, Pour PM, Saruc M, Iki K, Pour PM. Morphometric studies in human pancreatic cancer argues against the etiological role of type 2 diabetes in pancreatic cancer. Histology & Histopathology. 2010;25:423–32. doi: 10.14670/HH-25.423. [DOI] [PubMed] [Google Scholar]
- 142.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–11. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, Petersen GM. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Satake K, Takeuchi T, Homma T, Ozaki H. CA19-9 as a screening and diagnostic tool in symptomatic patients: the Japanese experience. Pancreas. 1994;9:703–6. doi: 10.1097/00006676-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 145.Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19:182–6. doi: 10.1111/j.1440-1746.2004.03219.x. [DOI] [PubMed] [Google Scholar]
- 146.Huang H, Dong X, Kang MX, Xu B, Chen Y, Zhang B, Chen J, Xie QP, Wu YL. Novel blood biomarkers of pancreatic cancer-associated diabetes mellitus identified by peripheral blood-based gene expression profiles. Am J Gastroenterol. 2010;105:1661–9. doi: 10.1038/ajg.2010.32. [DOI] [PubMed] [Google Scholar]
- 147.Stein KB, Snyder CF, Barone BB, Yeh HC, Peairs KS, Derr RL, Wolff AC, Brancati FL. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Digestive Diseases & Sciences. 2010;55:1839–51. doi: 10.1007/s10620-009-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu CH, Wu TY, Li CC, Lui MT, Chang KW, Kao SY. Impact of diabetes mellitus on the prognosis of patients with oral squamous cell carcinoma: a retrospective cohort study. Annals of Surgical Oncology. 2010;17:2175–83. doi: 10.1245/s10434-010-0996-1. [DOI] [PubMed] [Google Scholar]
- 149.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, Fuchs CS. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–40. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 150.Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, Kantarjian HM, O'Brien SM. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100:1179–85. doi: 10.1002/cncr.20071. [DOI] [PubMed] [Google Scholar]
- 151.Wolf I, Sadetzki S, Gluck I, Oberman B, Ben-David M, Papa MZ, Catane R, Kaufman B. Association between diabetes mellitus and adverse characteristics of breast cancer at presentation. Eur J Cancer. 2006;42:1077–82. doi: 10.1016/j.ejca.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 152.Richardson LC, Pollack LA. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 153.Ganti AK, Potti A, Koch M, Tendulkar K, Hanekom D, Koka V, Levitt R. Predictive value of clinical features at initial presentation in pancreatic adenocarcinoma: a series of 308 cases. Med Oncol. 2002;19:233–7. doi: 10.1385/MO:19:4:233. [DOI] [PubMed] [Google Scholar]
- 154.Kim TD, Oh HJ, Kim KH, Kim SM, Kim JH, Jang BI, Kim TN, Chung MK. Clinical characteristics of pancreatic cancer according to the presence of diabetes mellitus. Korean J Gastroenterol. 2004;43:35–40. [PubMed] [Google Scholar]
- 155.Pedrazzoli S, Sperti C, Pasquali C. Prediction of resectability and of surgical risk in pancreatic carcinoma; conditioning factors of survival after resective intervention. Chir Ital. 1994;46:30–8. [PubMed] [Google Scholar]
- 156.Andren-Sandberg A, Ihse I. Factors influencing survival after total pancreatectomy in patients with pancreatic cancer. Ann Surg. 1983;198:605–10. doi: 10.1097/00000658-198311000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kang SP, Saif MW, Kang SP, Saif MW. Clinical outcome of pancreatic cancer patients with diabetes mellitus: is diabetes a poor prognostic factor?. Jop: Journal of the Pancreas [Electronic Resource]; Highlights from the "2010 ASCO Annual Meeting"; Chicago, IL, USA. June 4-8, 2010; 2010. pp. 334–5. [PubMed] [Google Scholar]
- 158.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–62. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McWilliams RR, Matsumoto ME, Burch PA, Kim GP, Halfdanarson TR, de Andrade M, Reid-Lombardo K, Bamlet WR. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116:5054–62. doi: 10.1002/cncr.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li D, Abbruzzese JL. New strategies in pancreatic cancer: emerging epidemiologic and therapeutic concepts. Clin Cancer Res. 2010;16:4313–8. doi: 10.1158/1078-0432.CCR-09-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]