Abstract
Objective
Several interventions promote axonal growth and functional recovery when initiated shortly after CNS injury, including blockade of myelin-derived inhibitors with soluble Nogo Receptor (NgR1, RTN4R) ‘decoy’ protein. We examined the efficacy of this intervention in the much more prevalent and refractory condition of chronic spinal cord injury.
Methods
We eliminated the NgR1 pathway genetically in mice by conditional gene targeting starting 8 weeks after spinal hemisection injury and monitored locomotion in the open field and by video kinematics over the ensuing 4 months. In a separate pharmacological experiment, intrathecal NgR1 decoy protein administration was initiated 3 months after spinal cord contusion injury. Locomotion and raphespinal axon growth were assessed during 3 months of treatment between 4 and 6 months after contusion injury.
Results
Conditional deletion of NgR1 in the chronic state results in gradual improvement of motor function accompanied by increased density of raphespinal axons in the caudal spinal cord. In chronic rat spinal contusion, NgR1 decoy treatment from 4–6 months after injury results in 29% (10 of 35) of rats recovering weight-bearing status compared to 0% (0 of 29) of control rats (P<0.05). Open field BBB locomotor scores showed a significant improvement in the NgR-treated group relative to the control group (P<0.005, repeated measures ANOVA). An increase in raphespinal axon density caudal to the injury is detected in NgR1-decoy-treated animals by immunohistology and by positron emission tomography using a serotonin reuptake ligand.
Interpretation
Antagonizing myelin-derived inhibitors signaling with NgR1 decoy augments recovery from chronic spinal cord injury.
INTRODUCTION
Spinal cord injury (SCI) causes profound and persistent neurological deficits, many of which result from the interruption of axonal connectivity. A small number of compounds have been identified that when administered within the first few days subsequent to SCI either limit the extent of cell death following injury to lessen the resulting deficit, or promote the regrowth and/or collateral sprouting axons to restore functional connectivity, or both1, 2. The regrowth of axons that ‘bridge’ the lesion and the collateral sprouting of injured as well as uninjured fibers rostral and caudal to the lesion play critical roles in determining the extent of neurological recovery3, 4. Recent studies have emphasized a tight correlation of sprouting with functional recovery even when frank regeneration is absent5–8. Clinical trials are now testing the efficacy of several of these compounds in acute SCI1.
Because SCI frequently occurs in young adults and improved supportive care has dramatically increased life expectancy, the prevalence of chronic SCI is more than twenty times the annual incidence of acute SCI9. Neuroprotective strategies cannot benefit pre-existing chronic SCI, and strategies that promote axonal growth have shown efficacy only when administered shortly after injury. Thus, the prevailing model posits that the benefit of promoting axonal extension and collateral sprouting by blocking extrinsic inhibitors or enhancing intrinsic activators is restricted to the acute stage of injury following trauma, when axons are predicted to exhibit the highest potential for anatomical plasticity10. However, a recent study demonstrated that extensive regrowth of injured axons across the lesion site can be induced long after injury10. This approach required a combination of peripheral nerve injury, viral injection and cell transplantation. Unfortunately, and perhaps unexpectedly, this regrowth did not result in any functional benefit. Thus, axons in the chronic stage post-injury did regrow, but the extension of severed axons alone is insufficient to restore CNS function10. Alternative cell-based multi-modal therapies that include a component for blocking extrinsic inhibitors have also shown some success in specific chronic CNS injury models11, 12. However, pharmacological therapy for axonal growth with improved function in chronic SCI remains an unattained goal.
Myelin limits axonal growth and neurological recovery after SCI2. Amongst the inhibitory myelin proteins are Nogo-A (Rtn4A), myelin associated glycoprotein (MAG, Siglec-4) and oligodendrocyte myelin glycoprotein (OMgp). Each exhibits affinity for the Nogo-66 receptor (NgR1, Rtn4R), a protein detectable on axons13. The Nogo mutant phenotype has been controversial, and depends on the strain background14 and the lesion model15 as well as the specific allele7, 15–17, which is crucial to avoid chronic compensatory effects from other loci (Cafferty and Strittmatter, unpublished observations). For those models with a detectable Nogo-A phenotype, combined deletion of Nogo-A, MAG and OMgp allows substantial recovery from spinal cord trauma, greater than deletion of Nogo-A alone and much greater than deletion of MAG and OMgp7, 14, 15, 18, 19. For the receptor, constitutive deletion of NgR1 expression allows enhanced recovery after spinal cord injury or stroke, and permits sprouting and regenerative growth of raphespinal and rubrospinal axons20, 21. Sprouting, but not regeneration, of corticospinal fibers is observed15, 20–22. The Nogo, MAG and OMgp ligands also bind Paired-immunoglobulin-like receptor B (PirB) to inhibit axonal growth in vitro23. However, PirB mutant mice do not display improved recovery from CNS injury in any model yet tested24, 25.
The Nogo/NgR1 pathway has been targeted pharmacologically in acute and subacute CNS injury models by various methods. Anti-Nogo-A antibodies bind one myelin-associated inhibitory ligand (Nogo-A) and improve recovery when administered to rodents with spinal hemisection or stroke within one week of injury26–28. Similar humanized versions of these antibodies are now completing Phase I clinical trials29. An antagonist peptide, NEP1–40, selectively blocks Nogo-66 binding to NgR1 and promotes recovery from dorsal hemisection of thoracic spinal cord30, 31, lateral hemisection of cervical cord32 and stroke33, 34. One NEP1–40 study observed only slight increase in sprouting and inconsistent behavioral improvements35. Greatest benefit is obtained by neutralization of all three myelin inhibitors with a soluble truncated NgR1 fusion protein, NgR1(310)ecto-Fc36. Infusing this NgR1 decoy protein into the CNS within a week of spinal cord dorsal hemisection, stroke, spinal cord contusion or dorsal rhizotomy increases axonal growth responses and improves behavioral recovery21, 37–39. Downstream of NgR1, RhoA and ROCKII mediate myelin inhibition of axonal growth, and a RhoA inhibitor, ROCK inhibition and ROCKII gene deletion all promote functional recovery after acute SCI40–44. Critically, none of these interventions, nor any other pharmacological therapy to promote anatomical repair, has been reported to support neurological improvement when administered in the chronic stage post-injury after endogenous tissue responses have stabilized and the natural history is static.
We reasoned that because many axonal tracts and intrinsic spinal circuits remain intact in regions proximal and circumferentially to SCI sites and are continually exposed to myelin and myelin debris, blockade of myelin inhibitors long after spinal injury might stimulate axonal growth and recovery. We utilized a drug-regulated conditional deletion of NgR1 and a pharmacological treatment with an NgR-Fc decoy protein, AA-NgR(310)ecto-Fc, to test this hypothesis. Here, we provide evidence that blockade of myelin inhibition allows raphespinal axon growth and enhances recovery in chronic SCI. In addition, we describe a clinically adaptable in vivo imaging method to monitor raphespinal axon growth stimulated by NgR decoy protein following chronic spinal contusion injury. Together, these data support a role for pharmacological interventions targeting myelin-associated inhibitors and their receptors as a treatment for chronic SCI.
METHODS
Gene targeting and mouse strains
The multiple cloning site of pBluescriptII-KS(+) was replaced with a combination of unique restriction sites to facilitate the sequential construction of the targeting construct. The complete plasmid comprises (in order) a 6.6 Kb flanking arm of genomic sequence 5′ to a loxP site introduced at a Xho-I restriction site proximal to the 5′ end of exon 2 of NgR1, a pGK-NEO cassette flanked by FRT sequences approximately 500nt 3′ to the predicted end of the NgR1 mRNA, a second loxP site followed by the 86 nt preceding the splice acceptor sequence and first 12nt of exon 2 fused in-frame with eGFP, including an SV-40 polyadenlyation sequence, a 4 Kb flanking arm of genomic sequence and a negative selection cassette containing HSV-thymidine kinase. The plasmid containing the targeting construct was linearized at a unique Sfi-I site and electroporated into ES cells. ES cells were selected using G418 and FIAU. Chimeric mice were generated and crossed first onto the C57BL/6J strain and then a C57BL/6J strain expressing the Flp recombinase ubiquitously (Flp deleter strain). Progeny of this cross was screened by PCR to confirm the removal of the pGK-NEO cassette.
The estrogen-regulated ubiquitous cre recombinase mouse strain pActin-ER-Cre (B6.Cg-Tg(CAG-cre/Esr1)5Amc/J)45 was obtained from The Jackson Laboratory. The constitutive mouse strain pActin-Cre (Tg(CAG-cre)1Nagy) was provided by the Yale Animal Genomics Services Center. Tamoxifen was solubilized in corn oil to 10mg/ml by heating to 37°C and sonicating repeatedly. The drug was administered at a dose of 100mg/kg by intraperitoneal injection for three consecutive days. Mice were backcrossed to C57Bl6J for >6 generations.
Southern blot
Genomic DNA was prepared from cerebral cortex and hippocampus. Genomic DNA was digested with Hind-III, separated by electrophoresis, and blotted onto nylon membranes (Brightstar-Plus, Ambion). The NgR1 probe was amplified with the following primers: NR1 forward, 5′-TTC AGA TGT GTG GTT TTG GTG ACC-3′ (beginning at nt 221 5′ to the beginning of exon 2), and NR1 reverse: 5′-GAA GGT GAG GAG GAA GAG AGG GAG-3′ (ending at nt 721 5′ to the beginning of exon 2). The GFP probe corresponded to 400 nt of eGFP amplified with GFP forward 5′-GGC GAG GGC GAT GCC ACC TAC GGC-3′ (beginning at nt 100 after the start ATG), and GFP reverse 5′-GCG GAT CTT GAA GTT CAC CTT GAT GCC-3′ (ending at nt 507 after the start ATG). The purified probe template was biotinylated with the BrightStar Psoralen-Biotin Kit (Ambion). Blots were hybridized overnight with denatured probe at 68°C. After washing, bound probe was detected with the BrightStar Biodetect Kit and autoradiography film.
Northern blot
Cerebral cortex and hippocampus from one hemisphere were homogenized in 20 volumes (w/v) of Trizol Reagent (Invitrogen), aliquoted, frozen in liquid nitrogen and stored at −80°C. Thirty micrograms of total RNA was isolated and then separated on a 1% formaldehyde-agarose gel and blotted to nylon membrane (Brightstar-Plus, Ambion). Probes were prepared with the BrightStar psoralen-biotin nonisotopic labeling kit (Ambion) as described for Southern blotting (above). The NgR1 probe was prepared by first amplifying the cDNA sequence corresponding to the ~600nt carboxyl-terminal half of NgR1 with the following primers: NR1 forward, 5′-CAG TAC CTG CGA CTC AAT GAC AAC CCC-3′ (beginning at nt 757 relative to the start ATG), and NR1 reverse: 5′-CTT CCG GGA ACA ACC TGG CCT CC-3′ (ending at nt 1266 relative to the start ATG). The GFP probe is identical to the probe used for Southern blotting. After hybridization, bound probe was detected with the BrightStar Biodetect Kit.
Immunoblots
Cerebral cortex and hippocampus were homogenized in 20 volumes (w/v) of 100 mM NaCl, 10 mM Tris-Cl pH 7.4 and Triton X-100 and SDS were added to 1% and 0.5%, respectively. After centrifugation at 20,000 X g for 15 min, the supernatant was collected and assessed by immunoblot with antibodies directed against either NgR1 (goat anti-mouse NgR1, R&D systems) or GFP (rabbit anti-GFP, Sigma). Immunoreactivity was detected with horseradish peroxidase (HRP)-coupled secondary antibodies (Jackson Immunoresearch) and chemiluminescence (Pierce).
Optic Nerve Crush Injury and Regeneration
Cohorts of mice were administered tamoxifen 7–9 days prior injury, as described above. To crush the optic nerve, mice at 8–10 weeks of age were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a stereotactic apparatus. Topical 2% Lidocaine anesthetic was applied to the eyeball. The right optic nerve was exposed intra-orbitally with care taken to avoid damage to the ophthalmic artery. The nerve was injured by crushing with a jeweler’s forceps (Dumont #5, Fine Science Tools) for 10 seconds at a location 1 mm posterior to the eyeball. Two weeks thereafter, animals were anesthetized with an overdose of anesthetic and perfused trans-cardially with PBS followed by 4% paraformaldehyde. The optic nerves and retinas were dissected and post-fixed in 4% paraformaldehyde for 48 hours. The specimens were then transferred to 30% sucrose for cryoprotection, embedded in OCT and cryo-sectioned in the longitudinal plane at 16 μm thickness. The sections were stained with anti-GAP-43 antibody (1:1,000) followed by a fluorescent-tagged donkey anti-sheep (IgG) secondary. Four longitudinal sections of optic nerve were selected from each animal. The number of GAP-43 positive axons was counted at 0.5 mm and 1 mm distal to the lesion site. The total number of regenerating axons was calculated from these counts (Leon et al, 2000). The retina was also examined for ganglion cell survival using anti-βIII tubulin antibody. No differences were detected between different nerve crush groups (data not shown).
Chronic Thoracic Dorsal Hemisection Injury
Dorsal hemisections were performed on mice by a surgeon blind to the cre transgene genotype using methods and post-operative care as described previously7, 18. All mice had BMS scores of ≤ 4 at 75 days post-injury prior to tamoxifen treatment by the method described above.
Chronic Rat Spinal Contusion injury with Prolonged Intracerebroventricular Therapy
Female Sprague-Dawley rats (250–270 g, 11–12 weeks of age, n=70) were anesthetized with intraperitoneal injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). A laminectomy was conducted at the caudal portion of T6 and all of T7 spinal levels. A T7 moderate contusion injury (10 g, 25 mm) was produced with the MASCIS impactor 37, 46, 47. After the contusion, locomotor performance was assessed at one-week intervals using the BBB score in the open field. Locomotor performance stabilized at a score of 8 by 7 weeks after injury.
An intracerebroventricular cannula was used for AA-NgR(310)ecto-Fc therapy. To avoid any confounding unfavorable effect of the cannula implantation procedure on locomotor scores, a cannula (Alzet brain infusion kit II; Alza Scientific Products) was implanted into the right lateral ventricle at 10 weeks post-contusion injury as described previously 21, 48. Briefly, the scalp was opened, a burr hole was drilled through the skull, and a cannula was introduced into the right lateral ventricle at stereotaxic coordinates 0.6mm posterior and 1.2mm lateral to bregma and 4.0mm deep relative to the pial_surface. The cannula was connected to an osmotic minipump containing PBS placed subcutaneously over the scapulae. The cannula was fixed in place with cyanoacrylate, and the skin was sutured.
Two weeks after cannula implantation (12 weeks post-contusion injury), the rats were reanesthetized and the minipumps were replaced with new osmotic minipumps (Alza Scientific Products) connected to the same cannula. These pumps delivered 2.5 μl/hour for 28 days and were filled with 2.25 mg AA-NgR(310)ecto-Fc (0.29 mg/kg/day) or 2.25 mg IgG from rat serum (#14131–50 mg, Sigma) in 2ml PBS. A total of 64 rats survived from contusion injury to 12 weeks and were entered with into the treatment randomization. Rats were randomly assigned to one of the two groups, and the surgeon was unaware of the assignment. The duration of treatment was 12 weeks. A new osmotic mimipump filled with same amount of AA-NgR(310)ecto-Fc or rat IgG was switched every 4 weeks.
AA-NgR(310)ecto-Fc protein
Purified rat protein was produced in CHO cells and purified as described previously 38 with one modification. Because the wild fusion protein exhibits a high percentage of disulfide bond heterogeneity, a variant was produced in which the two Cys residues at position 266 and 309 of the full length NgR1 were changed to Ala. This AA variant is homogenous with respect to disulfide bonding and is fully active in vitro 49.
Behavioral Testing
For mouse behavioral observation, the BMS locomotor scale was used 50. For the rat behavioral testing, the BBB locomotor scale was used 46, 51. All behavioral tests were performed by two researchers unaware of the genotype of the mice or of the identity of the compound in the minipump. Observations were made once per week. Grip strength was measured using a Columbus Instruments force meter for a random subset of animals. Each rat was tested three times for each limb and the data were averaged.
Limb Kinematics
One week before initiation of treatment (tamoxifen or AA-NgR(310)ecto-Fc protein), and several months later near the end of treatment, mice or rats were videorecorded while locomoting to the end of a one meter plexiglass track. Prior to video monitoring, reflective markers (B&L Engineering) were attached using non-toxic glue bilaterally to: the iliac crest; the head of the humerus; the greater trochanter of the knee; the lateral malleolus; the fifth metatarsal; and the third toe. Recordings were made from four synchronized cameras at 100 Hz (Basler Vision Technologies) placed at approximately 45° and 135° relative to the position of the track. We utilized the SIMI motion-capture software (SIMI Reality Motion Systems) to obtain three-dimensional coordinates of the markers during locomotion. We modeled the hind limb as an interconnected chain of rigid segments. We then extrapolated multiple gait parameters from recordings containing at least three consecutive step cycles.
Data was exported using the SIMI motion-capture software numerically as Excel (Microsoft Corporation) spreadsheets as well as individual frame-by-frame images of gait cycles of each animal. We analyzed the position of the toe relative to distance traveled in the y (anterior-posterior) plane and z plane (superior-inferior), after normalizing the position of the toe relative to a fixed iliac crest (rat) or hip (mouse) in order to analyze the swing phase of the gait cycle. Additional measures were the angle of the ankle joint and the absolute velocity of the foot as functions of time. The gait cycle was defined as a single attempted forward excursion by the hindlimb as many of the animals were unable to step due to the nature of the injury. Frame by frame images were analyzed using ImageJ (National Institutes of Health) and Illustrator (Adobe Systems).
Histology and Analysis
Animals were perfused transcardially with PBS, followed by a 4% paraformaldehyde/PBS solution. The spinal cord 10 mm rostral to and 10 mm caudal to the lesion center was embedded in a glutaraldehyde-polymerized albumin matrix and cut parasigitally in the thickness of 40 μm on a vibratome. Transverse sections (40 μm) were collected from the spinal cord 11 to 16 mm rostral to and 11 to 16 mm caudal to the lesion center 15, 18, 20, 30, 31, 37, 38, 52, 53.
Sagittal sections of thoracic spinal cord were incubated with anti–5-hydroxytryptamine (anti–5-HT) antibody (1:10,000; Immunostar, Hudson, WI) and then with Alexa568-labeled secondary antibody (Invitrogen) to detect raphespinal fibers15, 18, 20, 30, 31, 37, 38, 52, 53. Transverse sections 11–16 mm caudal to the lesion center were incubated with anti–5-hydroxytryptamine (anti–5-HT) antibody or anti-serotonin transporter antibody (1:10,000 or 1:1,000; Immunostar, Hudson, WI) and were visualized with appropriate secondary antibody conjugated to AlexaFluor 568 (Invitrogen).
Image analysis was performed with National Institutes of Health (NIH) image version 1.62, as described previously15, 18, 20, 30, 31, 37, 38, 52, 53. For analysis of serotonin innervation, immunoreactive serotonin fibers in the ventral horn of transverse sections caudal to the lesion center were selected by thresholding; then the length of serotonin fiber per area was measured after using the ‘skeletonize’ function. For camera lucida tracing of 5-HT–immunoreactive fibers, 10 serial sections at 200μm intervals from each animal were photographed digitally, and fibers were traced using on a computer using Adobe Photoshop 7.0 software (Adobe Systems, Mountain View, CA).
Positron Image Tomography with [11C]AFM
Cohorts of injured rats were imaged twice, once at 12 weeks post spinal contusion prior to treatment, and again after 12 weeks of treatment. Uninjured control rats or complete transection rats were imaged once. Rats were anesthetized by isoflurane inhalation, and then 50 ± 30 MBq of [11C]AFM ([11C]2-[2-(dimethylaminomethyl)phenylthio]-5-fluoromethylphenylamine) was administered in sterile saline by tail vein injection (injected mass: 0.12 ± 0.09 μg). After injection, dynamic PET images were acquired on an HRRT scanner for 2 hours, as described54, 55. Images were collected from three rats simultaneously in each imaging session, and radioactivity concentration was assessed in the spinal cord at the cervical and lumbar enlargements. A subset of rats was imaged twice with [11C]AFM, once under baseline condition, and once after receiving citalopram, a serotonin transporter inhibitor (2 mg/kg in saline) 15 min prior to the second [11C]AFM PET scan to block specific binding sites. Non-specific uptake (~30% of cervical uptake) detected in the presence of citalopram was subtracted form the total uptake, to assess [11C]AFM specific binding signal.
RESULTS
Conditional Gene Targeting of the ngr1 Locus
To test definitively whether loss of NgR1 function in the chronically injured state might yield functional benefit we generated a conditionally targeted NgR1 allele, ngr1flox, by flanking the second exon (containing the entire coding sequence for the mature protein) with loxP sites (Suppl. Fig. S1A). In mice harboring a transgene of cre recombinase (Cre) driven by the constitutive β-actin promotor, recombination of the NgR1 allele is essentially complete (Suppl. Fig. S1B). To provide temporal control over deletion, we utilized a transgene with this promotor that drives expression of a fusion protein of Cre and a mutant version of the estrogen-receptor (ER)45. Tamoxifen treatment leads to highly efficient NgR1 gene rearrangement and near total loss of mRNA and protein in the adult CNS within a week (Fig. 1A, Suppl. Fig. 1B–E). Prior to recombination, this allele expresses NgR1 protein at WT levels (Fig. 1A, Suppl. Fig. 1C–E). After Cre-mediated recombination, this allele produces detectable GFP mRNA and protein. GFP expression levels are not sufficient to permit visualization of intrinsic GFP fluorescence but can be detected with signal amplification.
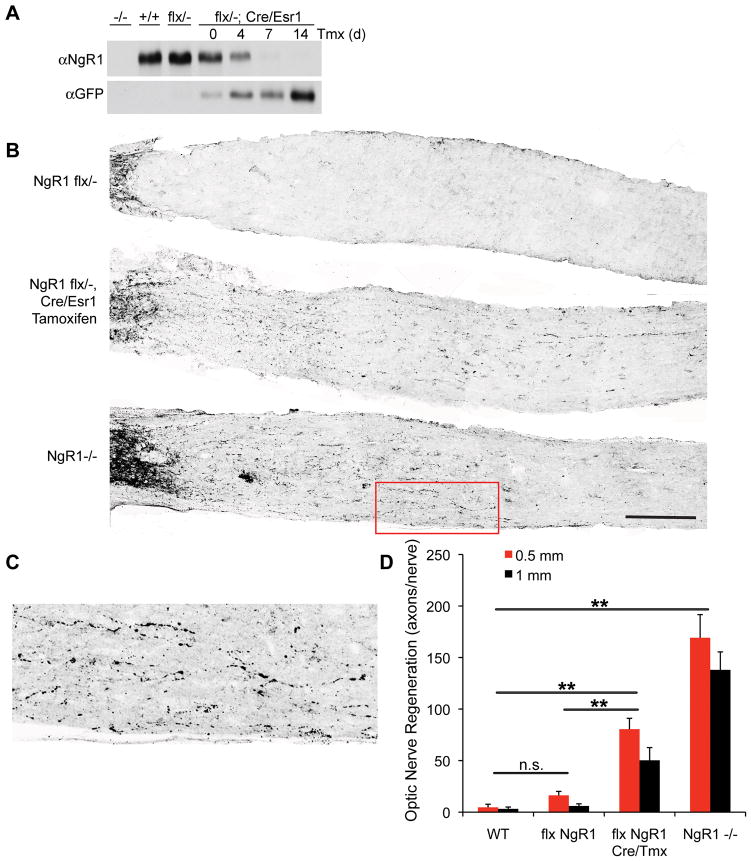
Figure 1. Recovery from Chronic SCI Deficits after NgR1 Deletion.
A, Cre-mediated recombination from the pActin-Cre/Esr1 transgene abolishes expression of NgR1 following tamoxifen injection. Immunoblots of brain lysate from mice transgenic for pActin-Cre/Esr1 at several time points after tamoxifen injection to activate the Cre fusion protein with antibodies directed against either NgR1 or GFP.
B, Mice of the indicated genotypes underwent optic nerve crush injury at 10–12 weeks of age, and tissue was collected 14 days later for anti-GAP-43 immunohistology of injured fibers. The flx NgR1 mice carrying the cre/Esr1 transgene were treated with tamoxifen one week prior to injury. Intact fibers close to the eye are visible at the far left, and regeneration past the lesion is detected in the bottom two panels. Scale bar, 250 μm.
C, Higher magnification view of the area boxed in red of B demonstrates regenerating fibers in NgR1−/− mice.
D, The number of regenerating optic nerve fibers is presented as a function of distance central to the crush site and of genotype. Data are mean ± sem for 4–8 mice per group. **, P<0.01, one-way ANOVA with pairwise post-hoc Fisher’s least-significant difference test, SPSS. The indicated comparisons are valid at both the 0.5 mm and the 1 mm distances.
To assess the functional completeness of NgR1 loss following tamoxifen treatment, we employed an optic nerve regeneration model. Previous studies have demonstrated that expression of a dominant negative form of NgR1 in retinal ganglion cells with a recombinant virus yields a subtle axon regeneration phenotype that substantially synergizes with an enhancement of intrinsic growth potential by intraocular inflammation56. A constitutive null allele of ngr1 has a stronger axonal regeneration phenotype after optic nerve crush that is clearly detectable without zymosan injection or lens injury (Fig. 1B–D). We examined whether the conditional deletion of ngr1 would produce an axonal regeneration phenotype similar to the constitutive knockout. Axonal regeneration in ngr1flox/− mice carrying the Cre transgene and treated with tamoxifen one week prior to optic nerve crush resembles that in ngr1−/− mice and is significantly (P<0.01, ANOVA) greater than WT mice (Fig. 1B, D). Axonal regeneration is not as complete in the conditional allele as the constitutive deletion; this is likely to reflect the 7-day pretreatment providing subtotal gene rearrangement (Fig. 1A). For ngr1flox/− mice without Cre/tamoxifen, axonal regeneration was not significantly different from the low wild type levels (Fig. 1B, D). Thus, the conditional allele allows functional deletion of NgR1 in vivo by tamoxifen, and resembles the ngr1 null axonal regeneration phenotype.
Functional Improvement and Raphespinal Growth after Genetic Deletion of NgR1 in Chronic Mouse SCI
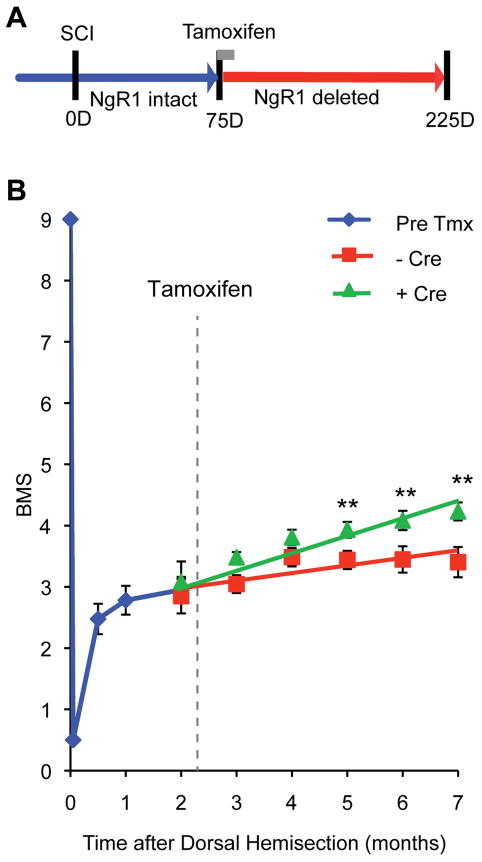
Next, we utilized the conditional ngr1 allele to assess recovery and axonal growth in the chronic post-injury state. Optic nerve regeneration is not a chronic injury model as the vast majority of retinal ganglion cells die within one month of injury57, 58. Instead, we subjected ngr1flox/− and ngr1flox/flox mice, of which 18 of 41 also carried the Cre/Esr1 transgene, to a spinal cord dorsal hemisection injury. Animals were then observed without intervention for 75 days, a duration greater than the time course of natural (limited) recovery (Fig. 2A). At 75 days, prior to tamoxifen treatment when NgR1 expression is at wild-type levels, no differences in locomotor performance are detected between these genotypes (BMS = 2.95±0.15 at two months in Fig. 2B). All mice were treated with tamoxifen at 75 days post-injury. Over the ensuing 125 days, mice expressing ER-Cre, and therefore lacking NgR1 expression, exhibited improved open field BMS locomotion scores (Fig. 2B). Over this time frame there was no significant improvement in the Cre-negative group (final BMS = 3.40±0.25) relative to the pre-tamoxifen performance (2 month BMS = 2.95±0.15). In contrast, the Cre-positive group improved significantly during the months post tamoxifen (final BMS = 4.25±0.15, P=0.03 by repeated measures ANOVA relative to Cre-negative, and P<0.01 at 5–7 months relative to the pre-tamoxifen 2 month value). On the BMS scale, a score of 3, the average starting point for both groups, represents plantar placing and/or dorsal stepping, but no plantar stepping. In comparison, a score of 4 or 5 indicates occasional or regular plantar stepping with weight support. Thus, Cre-mediated deletion of NgR1 expression in the chronic stage post-injury enabled affected mice to improve stepping performance. This improvement after NgR1 elimination was gradual over months, consistent with a chronic anatomical growth response as opposed to an acute biochemical event, which would have been evident within a week as NgR1 protein levels diminished.
Figure 2. Recovery of Locomotor Function in Chronic Spinal Cord Hemisection by NgR1 Deletion.
A, Schematic of mouse chronic SCI experiment. All mice carried flx NgR1 alleles, but one group also carried the Cre/Esr1 transgene to allow tamoxifen-induced recombination (Cre-positive, n=23) while the other group did not (Cre-negative, n=18). 75 days after SCI, mice received tamoxifen to abolish NgR1 expression.
B, The BMS score after the initiation of treatment is plotted. Recovery is greater in the Cre/Esr1 group, P=0.030 by repeated measures ANOVA for the effect of Cre after tamoxifen treatment. Data are mean ± sem. **, P<0.01, one-way ANOVA for the Cre-positive group relative to pre-tamoxifen value by Fisher’s least-significant difference test using SPSS software.
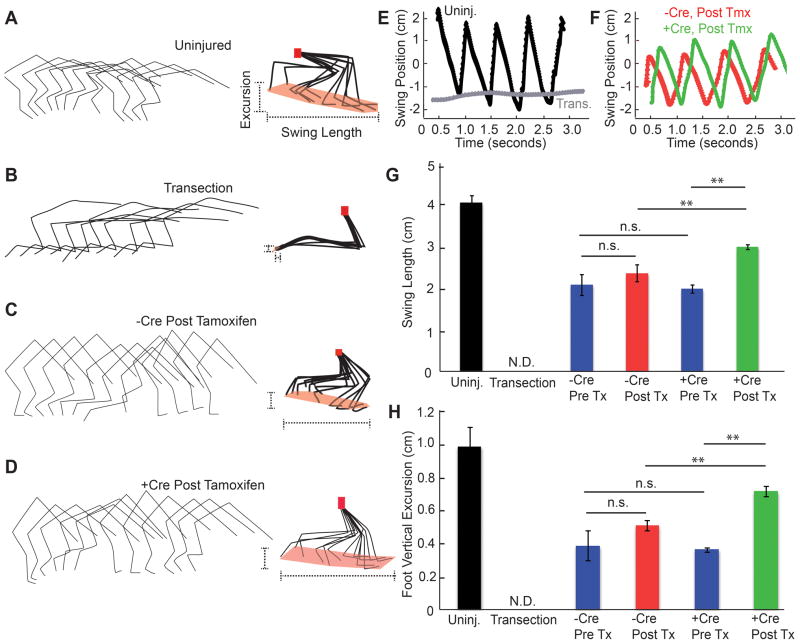
Stepping performance was also assessed with high-speed video limb kinematics (Fig. 3). Markers over each joint of both hindlimbs were tracked during unassisted locomotion to provide the most functionally relevant performance. Uninjured mice exhibit robust step cycles (Fig. 3A), while 10 days after a total thoracic transection there is nearly complete paralysis (Fig. 3B). In mice with intact NgR1 expression, unassisted gait remained significantly impaired 6–7 months after dorsal hemisection, as expected for chronic post-injury mice (Fig. 3C). We focused on measures of the length and height of foot swing with each step cycle for chronic dorsal hemisection mice both before and after tamoxifen administration. Prior to tamoxifen treatment, the Cre-negative and Cre-positive groups displayed similar deficits (Fig. 3G, H). The anterior-posterior length of the foot swing relative to the hip was reduced by half compared to uninjured control mice (Fig. 3A, C, E–G). The vertical height of the foot cycle relative to the hip was also reduced by 50% (Fig. 3A, C, H). After tamoxifen treatment, these limb kinematic measurements in the Cre-negative group at 6–7 months were comparable to pre-tamoxifen 75-day post-injury values, revealing a stable gait impairment, with no change in the length or height of foot swing relative to the hip (Fig. 3G, H). In contrast, the average height and length of foot excursions in Cre-positive mice increased during the 5 months after tamoxifen treatment. These values represent statistically significant improvements (P<0.01, one way ANOVA) relative to both the pre-tamoxifen values and to the Cre-negative group in which NgR1 expression is intact, but are significantly lower than the values for uninjured mice (Fig. 3D, F–H). Thus, foot movements remained restricted compared to uninjured mice, but improved after NgR1 deletion in chronically injured mice. The reproducibility of this phenotype is illustrated for 8 Cre-negative control and 8 Cre-positive mice (Suppl. Fig. S2). Other measures of the gait cycle, including the foot velocity and the ankle angle, are different in the Cre-positive mice (Suppl. Fig. S3). In particular, the ankle angle is more extended in the mice with NgR1 deletion (Cre-positive), and recovers after each step more quickly, than in the mice with NgR1 intact (Cre-negative).
Figure 3. Kinematic Analysis of Hindlimb Function after NgR1 Deletion in Chronic SCI.
A–D, Hindlimb kinematic analysis from individual mice with no injury (A), or a complete transection 7 days earlier (B) or dorsal hemisection 6 months earlier (C, D). The dorsal hemisection mice with flx NgR1 alleles were treated with tamoxifen 75 days after injury after injury and either carried Cre transgene (D) or not (C). Tracings of joint and limb position from one hindlimb during one gait cycle are shown for every 21st frame at the left. On the right, the foot, ankle and knees have been normalized to the hip position (red dots). The extent of foot swing is highlighted (dotted lines).
E, F, The anterior-posterior position of the foot relative to the hip from one limb of each of the indicated groups as in A-D is plotted as a function of time. Gait cycles are reflected in sequential peaks.
G, The length of the foot swing relative to the hip in the anterior-posterior dimension is shown for each group of mice. Mice were examined when uninjured, one week after complete spinal cord transection, or 10–35 weeks after dorsal hemisection injury. The Pre Tx groups were analyzed immediately before tamoxifen (10–11 weeks post injury), and the Post Tx groups were 4–5 months after tamoxifen (30–35 weeks post injury). Data are mean ± sem, n= 46 limbs for mice without Cre and n=36 for mice with Cre transgene. **, P<0.01, one-way ANOVA with pairwise post-hoc Fisher’s least-significant difference test, SPSS.
H, The vertical excursion of the foot relative to the hip is reported for the same groups of mice as in G. Data are mean ± sem. **, P<0.01, one-way ANOVA with pairwise post-hoc analysis using SPSS software.
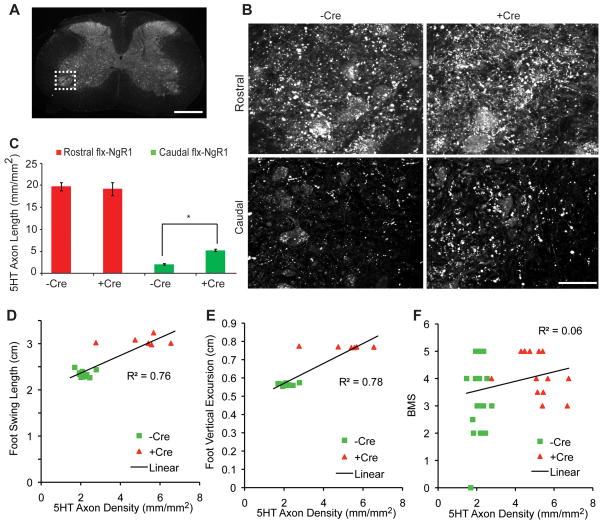
Previously, we observed that following a dorsal hemisection spinal cord injury in mice with a constitutive deletion of NgR1, the density of fibers from the descending raphespinal serotonergic system increases caudal to the lesion20. In this study, at the conclusion of this extended observation period, we examined the distribution of serotonergic fibers in the spinal cords from both Cre-negative and Cre-positive mice. In both groups, raphespinal fibers were substantially depleted in the ventral horn of the lumbar caudal spinal cord relative to the cervical enlargement (Fig. 4). Mice carrying the Cre transgene and thus lacking NgR1 beginning ~75 days after hemisection exhibit increased 5HT fiber density in the lumbar cord relative to Cre-negative controls (Fig. 4B, C). This increase may reflect axonal growth of spared fibers, long distance growth of cut fibers, or both. Therefore, axonal growth in one or several tracts may contribute to the slow improvement in motor function observed in Cre-positive mice. Indeed, there was a strong correlation of 5HT axon density with the parameters measured kinematically (Fig. 4D, E).
Figure 4. Raphespinal Growth Induced by NgR1 Deletion in Chronic SCI.
A, Low magnification transverse section of the thoracic spinal cord indicating the ventral horn region shown at high magnification in B (dotted lines). Scale bar, 500 μm.
B, Immunoreactive 5HT fibers in the ventral horn of the cervical (Rostral) and lumbar (Caudal) enlargements are shown from a mouse with or with the Cre transgene following dorsal hemisection and tamoxifen treatment at 35 weeks after injury. Scale bar, 50 μm.
C, The length of 5HT immunoreactive axonal length in B. Data are mean ± sem, n = 23 mice for –Cre and n= 18 mice for +Cre group. *, P<0.01, one-way ANOVA with pairwise post-hoc analysis using SPSS software.
D–F, Correlation between 5HT immunoreactive axonal length and the kinematic analysis from Fig. 3 or the BMS data from Fig. 2. Correlation coefficients from linear regression are shown.
Improved Locomotion and Axon Growth after Treatment of Rat Chronic Spinal Contusion with AA-NgR(310)ecto-Fc
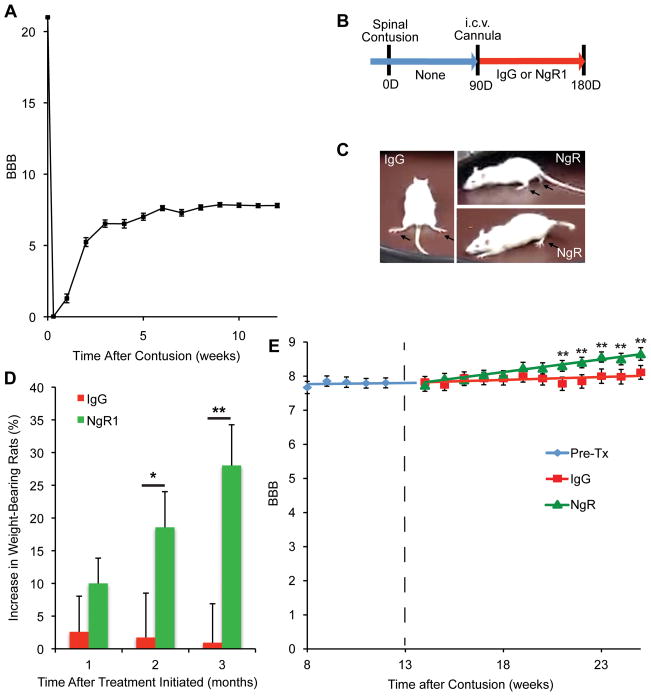
Based on these positive results with genetic perturbation of NgR1 expression in chronically injured mice, we next attempted a more clinically relevant pharmacological treatment of chronic spinal cord contusion in rats. A cohort of rats underwent mid-thoracic contusion; they were not treated for 3 months but hindlimb locomotor performance was evaluated weekly. The average BBB score at 12 weeks was 7.75 ± 0.10, n=64 (Fig. 5A), meaning that the majority of rats were capable of hindlimb movement, but not weight support46, 51. After introducing a intracerebroventricular (i.c.v.) cannula, rats were randomized to receive either AA-NgR(310)ecto-Fc or control IgG protein for 12 weeks at a dose of 0.29 mg/kg/d 21, 37, 38 (Fig. 4B). The initial BBB scores were identical in the two groups (Fig. 5A, E). Twelve weeks later, there was a trend towards improved hindlimb grip strength in rats treated with NgR1 decoy protein (hindlimb force as a percentage of forelimb force, 11.5±0.8% n=13 in IgG group assessed for grip strength, versus 14.5±2.2% n=12 in NgR group; P=0.11, one-tailed t test). However, the more conspicuous behavioral change was the conversion from hindlimb non-weight-bearing to hindlimb weight-bearing locomotion (Fig. 5C). The number of AA-NgR(310)ecto-Fc treated animals able to bear weight in the open field increased by ten (29% of 35 rats, P<0.05), while there was no significant increase in weight bearing in the control IgG group (29 rats) (Fig. 5D). During the treatment phase of the study, BBB scores showed a significant improvement in the NgR-treated group relative to the IgG group (P=0.002, by repeated measures ANOVA), while no significant improvement versus time was detected in the IgG group from a pre-treatment value of 7.75±0.10 to 8.10±0.20 post-treatment. BBB scores of the AA-NgR(310)ecto-Fc treated group were significantly improved between 8 to 12 weeks of treatment, at 21–25 weeks post-contusion (P<0.01, ANOVA, from 7.75±0.10 at 12 weeks to 8.65±0.20 at 25 weeks, Fig. 5E). Thus, AA-NgR(310)ecto-Fc treatment of chronic spinal contusion improves neurological recovery, in particular open field locomotion.
Figure 5. Recovery of weight bearing after NgR1 treatment of chronically spinal cord contused rats.
A, Spontaneous improvement of open field locomotion (BBB score) as a function of time after thoracic spinal cord contusion injury for all rats prior to randomization to IgG or NgR1 treatment. Mean ± sem, n = 64.
B, Schematic of experiment. Two weeks after the i.c.v. cannula implantation (12 weeks post-contusion injury), rats were assigned to one of two treatment groups. The PBS minipumps were replaced with new osmotic minipumps filled with 2.25 mg AA-NgR(310)ecto-Fc (0.29 mg/kg/day) or 2.25 mg rat IgG in 2 ml PBS. The duration of treatment was 12 weeks. A new osmotic mimipump filled with same amount of AA-NgR(310)ecto-Fc or rat IgG replaced each depleted pump every 4 weeks.
C, Examples of a control rat without weight support at the end of the treatment period and two of the seven AA-NgR(310)ecto-Fc treated rats that regained weight support.
D, The increase in the percentage of rats showing body weight support with at least one hindlimb as a function of time during therapy is reported. P=0.022 by repeated measures ANOVA for the effect of NgR1 versus IgG treatment, and *, P<0.05, and **, P<0.01 for the indicated comparisons by one-way ANOVA.
E, The course of recovery after the initiation of treatment (dotted line) is plotted as a function of time. The locomotor BBB scores from the IgG and AA-NgR(310)ecto-Fc-treated groups were indistinguishable at the initiation of treatment. Data are mean ± sem, n=29 for IgG and n=35 for NgR1 group. P=0.002 by repeated measures ANOVA for the effect of NgR1 versus IgG treatment, and **, P<0.01, one-way ANOVA for NgR1 treated values relative to pre-treatment value by post-hoc Fisher’s least-significant difference test using SPSS software.
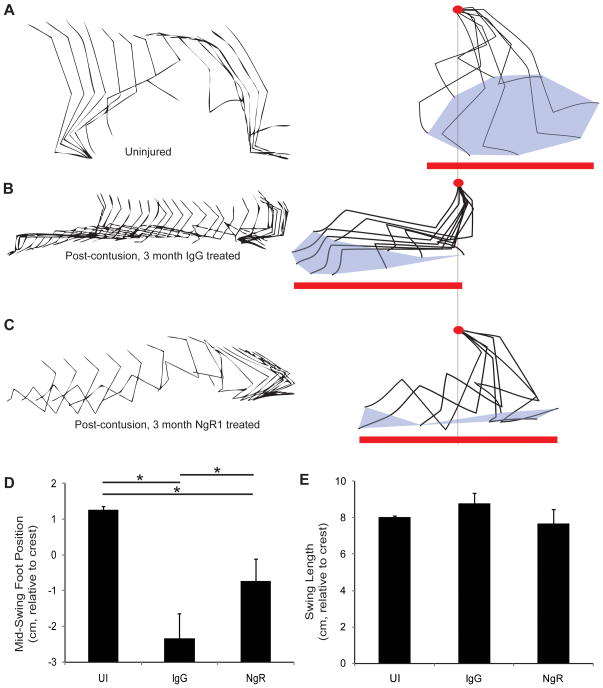
To provide quantitative measurements of open field locomotion, we also monitored limb kinematics in a randomly selected subset of control and AA-NgR(310)ecto-Fc treated rats (Fig. 6). In comparison to uninjured animals (Fig. 6A), the control group at 6 months post-contusion injury exhibits multiple deficits (Fig. 6B). First, while the length of the foot swing in the anterior-posterior dimension on each step relative to the iliac crest is maintained at near normal levels (Fig. 6E), the average foot position is shifted far posterior (Fig. 6D). This observation is in agreement with a previous study59. Second, uninjured rats produce a gait cycle in which the foot is centered under or anterior to the iliac crest, but the chronically-contused control rats show a range of foot position that remain posterior to the iliac crest at all times. In contrast, in the AA-NgR(310)ecto-Fc-treated group (Fig. 6C), there was significant normalization of foot position. The majority of AA-NgR(310)ecto-Fc-treated rats moved their foot anterior to the iliac crest with each cycle. The average position of the foot relative to the iliac crest resembled uninjured rats to a greater extent than it did the control injury group (Fig. 6D). The reproducibility of this result is illustrated for 5 IgG protein treated control and 5 AA-NgR(310)ecto-Fc-treated rats (Suppl. Fig. S4). Ankle flexion of the AA-NgR(310)ecto-Fc-treated rats is confined significantly more precisely to the swing phase of the gait cycle than for the IgG-treated rats (Suppl. Fig. S5). For the control IgG group, the ankle angle is extended 200 msec prior to maximal foot movement and remains flexed for 200 msec afterwards. In this sense, the NgR-treated rats resemble uninjured rats more closely than do the control injury group.
Figure 6. Gait kinematics in chronic SCI rats after NgR1 treatment.
A–C, Stick tracing of joint and limb position in the anterior-posterior plane (left), with forward to right, and every 16th frame shown. The limb positions after normalizing to the iliac crest position (red dot) are provided at right. The total extent of foot swing (red line) and the toe cycle (gray fill) are shown. The individual examples are of an uninjured animal (A), and of rats after treatment for 12 weeks with IgG (B) or with AA-NgR(310)ecto-Fc (C).
D, The mid position of the foot swing relative to the iliac crest during the gait cycle in the different treatment groups. Data are mean ± sem for n = 12–14 limbs per condition. UI, uninjured. *, P<0.05 by one-way ANOVA with post-hoc pairwise comparison, SPSS.
E, The anterior-posterior extent of foot movement relative to the iliac crest for the different treatment groups. Data are mean ± sem for n = 12–14 limbs per condition.
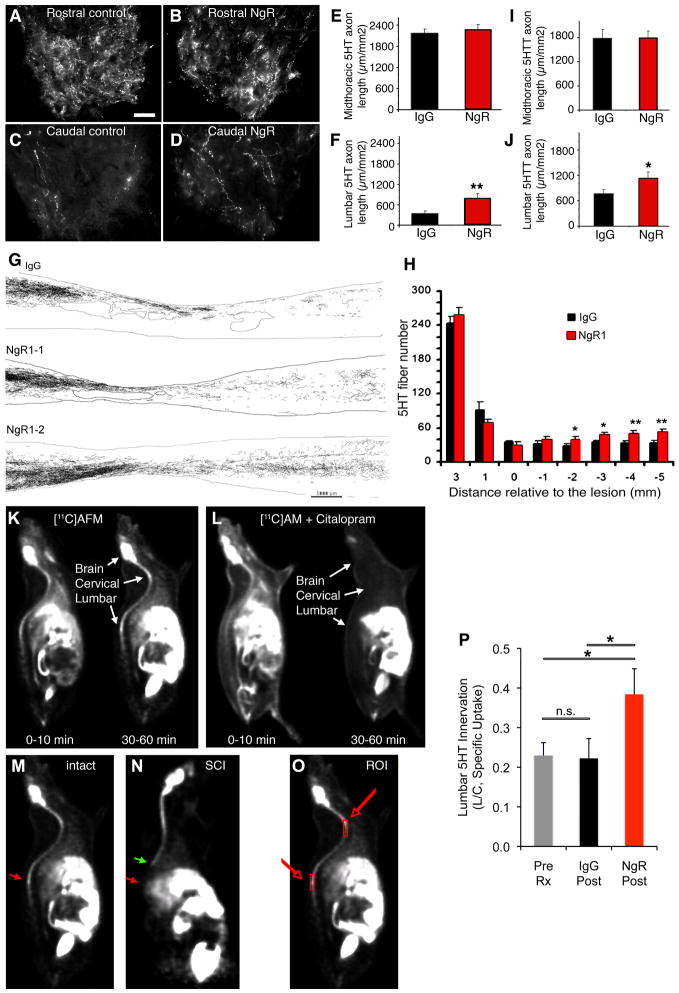
In previous studies examining the potential benefit of NgR1 antagonism following spinal cord injury, improved neurological function correlated with axonal growth16, 18, 20, 21, 37–39. Post-mortem histological examination of rats treated after acute spinal cord injury with NgR(310)ecto-Fc revealed an increase in the density of descending raphespinal axons caudal to the lesion site37, 38. Similarly, here we observed increased serotonin (5HT) fiber length in the lumbar spinal cord after treatment of chronic SCI (Fig. 7A–F). Camera lucida reconstructions of sagittal sections of thoracic spinal cord illustrate increased 5HT-immunoreative raphepspinal fibers throughout the caudal spinal cord of AA-NgR(310)ecto-Fc treated rats in comparison to IgG-treated controls (Fig. 7G, H). As expected, the degree of tissue loss at the injury site was not altered by AA-NgR(310)ecto-Fc treatment at this chronic stage (Suppl. Fig. S6). While there was a trend towards BDA-labeled corticospinal tract (CST) fibers extending into the lesion site, no CST fibers were detected in the spinal cord caudal to the lesion for either group (Suppl. Fig. S7), consistent with findings in subacute treatment of rat contusion injury with NgR(310)ecto-Fc37. These data provide histological evidence for axon growth from uninjured and/or injured fibers as a mechanism contributing to the behavioral improvement of rats treated with AA-NgR(310)ecto-Fc at a chronic stage following spinal contusion.
Figure 7. AA-NgR(310)ecto-Fc-induced raphespinal growth in chronic SCI.
A–D, Transverse section from the spinal cord 11–15 mm rostral or caudal to the contusion site were immunostained as indicated from rats completing 3 months of i.c.v. treatment. Anti-serotonin immunohistochemistry labels 5-HT fibers in the ventral horn of the spinal cord. Note the decreased innervation caudal to the injury and the partial recovery in the AA-NgR(310)ecto-Fc treated rats. Scale bar, 25 μm.
E, F, The length of 5HT-immunoreactive axon per unit area of ventral horn in the transverse plane was measured for chronic SCI rats completing 3 months treatment with the indicated proteins from micrographs as in A-D. The data are mean ± sem for n=21–25 per group, and the increase in the NgR1 treated group in the distal cord is significant; **, P<0.01, ANOVA.
G, Camera lucida drawings of serotonergic fibers from one IgG-treated and two AA-NgR(310)ecto-Fc-treated animals. Each drawing is a composite assembled from a set of 10 parasagittal sections spaced at intervals of 200 μm across the spinal cord. The contusion cavities are encircled near the center of each image. Increased numbers of serotonergic fibers are observed in the caudal spinal cord in the AA-NgR(310)ecto-Fc-treated (NgR1–1, NgR1–2) animals compared with the IgG-treated animals. Scale bar is 1000 μm.
H, Serotonergic (5HT) fiber number at various distances rostral and caudal to the center of the lesion from AA-NgR(310)ecto-Fc-treated (red bars) and control animals (black bars) is reported. *, P<0.05; **, P<0.01, ANOVA. For the x-axis, a positive value is rostral to the center of the lesion, and a negative value is caudal to the center of lesion.
I, J, Adjacent sections were processed for anti-serotonin transporter (5HTT) immunohistochemistry and measured as for 5HT in E, F. The data are mean ± for n=21–25 per group, and the increase in the NgR1 group in the distal cord is significant, P<0.05, ANOVA, *.
K, L, [11C]AFM radioactivity was visualized at the indicated times after an intravenous injection of tracer (50 ± 30 MBq, 0.12 ± 0.09 μg) with or without the pre-injection of the competitive ligand, citalopram (2 mg/kg, 15 min before [11C]AFM injection). The initial distribution of the tracer is broad with or without citalopram at 0–10 min. Specific uptake of [11C]AFM at 30–60 min in the brain, cervical spinal cord and lumbar spinal cord is blocked by citalopram.
M, N, A rat with an intact spinal cord was imaged with [11C]AFM and compared to a rat with a midthoracic transection one week earlier. Uptake at 30–60 minute is illustrated. The site of the lesion is indicated by the green arrow and the reduced uptake in the lumbar cord of the injured rat is indicated by the red arrow.
O, For quantitation of [11C]AFM uptake in the spinal cord, two regions of interest were selected as illustrated by the boxes on this intact rat image.
P, The ratio of lumbar to cervical [11C]AFM specific uptake at 30–60 minute post-tracer injection was determined in injured rats completing 3 months of treatment with control IgG or NgR(310)ecto-Fc, as illustrated in O. The data are mean ± sem for n=21–23 per group, and the increase in the NgR1 group in the distal cord is significant; *, P<0.05, ANOVA.
Positron Emission Tomography Imaging of Raphespinal Growth after NgR1 Decoy Treatment of Chronic Rat Spinal Contusion
Proof of concept clinical trials for chronic spinal cord injury would be greatly enhanced by methods to directly monitor the presence of axonal growth non-invasively. Diffusion tensor imaging by magnetic resonance can detect massive disruptions of highly fasciculated spinal cord tracts after injury, but it is extremely doubtful that this method can image the branched, tortuous and disorganized growth of descending raphespinal fibers that occurs after spinal cord injury in response to AA-NgR(310)ecto-Fc treatment 14, 16, 18, 20, 21, 37, 38, 60.
To visualize raphespinal axon growth in the caudal spinal cord in vivo, we evaluated imaging of presynaptic serotonin reuptake sites by Positron Emission Tomography (PET). One ligand, [11C]AFM, shows highly selective binding to serotonin transporters (SERT, 5HTT) in vivo54, 55. To verify the feasibility of such a PET ligand for use in detecting changes after spinal contusion, we first examined 5HTT-immunoreactivity in sagittal sections from injured animals and compared these results to our findings with 5HT immunostaining for raphespinal fibers. We observed an increase in 5HTT fiber length in the caudal spinal cord of NgR(310)ecto-Fc treated rats that was very similar to the distribution of 5HT immunolabeled fibers (Fig. 7I, J). Therefore, we obtained PET images from the spinal cord of anesthetized rats. The [11C]AFM tracer exhibits high uptake in the brain and spinal cord at 30–60 minutes post-injection (Fig. 7K). The fraction of [11C]AFM activity uptake attributable to nonspecific binding was determined by blockade with the competitive 5HTT inhibitor citalopram in healthy control animals. The ratio of standard uptake values (SUV = concentration normalized by activity dose and body weight) between citalopram and baseline scans was 0.35 ± 0.05 and 0.33 ± 0.05 for the cervical and lumbar cord, respectively (mean ± sem, n=5), i.e., more than 70% of the uptake at 30–60 min is specific binding. (Fig. 7K, L). The non-specific uptake fraction (30%) was then used to correct the total uptake values to specific uptake values. In rats with thoracic spinal cord complete transection, the cervical [11C]AFM specific binding signal is similar to that in uninjured animals but the signal in the lumbar enlargement is eliminated (Fig. 7M, N).
For spinal contusion rats prior to treatment at 3 months post-injury, an 80% reduction deficit in lumbar cord [11C]AFM specific signal is detected relative to cervical values (Fig. 7O–P). After treatment with NgR(310)ecto-Fc for 3 months, the lumbar specific uptake as a proportion of the cervical uptake was two-fold greater than in IgG-treated rats or in pretreatment rats (P<0.05, ANOVA; Fig. 7O–P), matching immunohistological results with either 5HT or 5HTT (Fig. 7I, J). Thus, PET imaging of 5HTT with [11C]AFM provides a non-invasive method to monitor serotonergic axonal growth after treatment with the axonal growth promoting compound, AA-NgR(310)ecto-Fc.
DISCUSSION
The major finding of the present work is that axon growth and neurological improvement can be achieved with a single molecular intervention in the chronically injured adult CNS. We utilized two different interventions in two different SCI model systems to verify that NgR1-directed intervention is effective many months after thoracic spinal cord injury. In mice, we generated a conditional allele of NgR1 and employed a drug-regulated Cre-dependent deletion strategy to study the role of NgR1 in limiting recovery at a chronic stage following dorsal hemisection SCI. In rats, we utilized intracerebral infusion to evaluate the effects of an NgR1 decoy receptor at a chronic stage after contusion SCI. In both systems, months after thoracic injury, intervention significantly improved open field locomotion, hindlimb kinematics, and caudal raphespinal axon density. These data provide the first demonstration that a directed molecular intervention has preclinical efficacy for improving mammalian neurological function in the chronic stage of CNS damage.
In previous analyses of chronic spinal cord injury, cell-based combination therapies have yielded some benefit. Cellular components have included olfactory ensheathing cells, Schwann cells, fibroblasts, neural stem cells and mesenchymal stem cells10–12. To achieve optimal benefits, cellular transplantation has been combined with a range of interventions, including modifications of cAMP level, surgeries to promote the intrinsic growth stage of the neuron, and neurotrophic factors. None of these interventions have produced significant neurological benefit for chronic SCI in the absence of surgical transplantation. In contrast to chronic SCI, several pharmacological treatments without surgical or cell-based intervention are beneficial in acute or subacute models of SCI. Pharmacological treatments targeting myelin-derived inhibitors, chondroitin sulfate proteoglycans, intracellular RhoA signaling cascades, cAMP levels, and neurotrophic factors, are efficacious when employed shortly after injury4. Similar to pharmacological intervention, perturbing the expression of single genes to promote axon growth and/or neurological improvement, including phosphatase and tensin homolog (PTEN)61, Nogo-A7, 8, 14, 15, 18, NgR120 and PTP-sigma62, 63, has only been effective when performed before or shortly after SCI. The current study establishes the possibility of pharmacological therapy alone as efficacious for chronic CNS injuries such as spinal cord trauma.
The locomotor and kinematic improvements observed in chronically-injured mice and rats after NgR1 perturbation emerge gradually and correlate with increased serotonin fiber density in the distal spinal cord. Most likely this reflects sprouting of raphespinal fibers. Treatment with the NgR1 decoy protein did not promote long distance regeneration of corticospinal fibers in the rat chronic contusion model. However, many lines of work support the model that short-range anatomical plasticity such as collateral sprouting can provide substantial functional benefit in the absence of long-distance axon regeneration. For example, the sprouting of uninjured corticospinal fibers after pyramidotomy and after ischemic stroke in constitutive mutant NgR1 mice correlate with functional recovery in the absence of regeneration15, 21. This restitution of function may depend in part from the recruitment of intraspinal poly-synaptic relays capable of providing substantial neurological replacement for injured long tracts5, 64. Thus, an absence of frank corticospinal regeneration does not imply absent restoration of functional polysynaptic circuits. Pharmacological interventions, such as ibuprofen and Y–27632, also promote axonal sprouting and functional recovery after spinal cord injury without long distance axonal regeneration41, 65. While the growth of raphespinal serotonin axons in the caudal spinal cord is driven by NgR1 intervention long after spinal cord injury, anatomical rearrangements of other injured and uninjured descending and propriospinal fiber tracts at multiple levels of neuraxis are probable contributors to the improvements in locomotion and kinematics observed in this study.
While sprouting or plasticity are likely to occur with interruption NgR1 pathways, the optic nerve regeneration studies with constitutive or conditional NgR1 deletion demonstrate that absence of this one protein in adult mice permits axonal regeneration per se in vivo. This finding extends previous studies using a dominant negative truncated NgR1 virus in combination with an induction of intrinsic growth potential by macrophage inflammation56. Presumably, the more prominent phenotype after genetic deletion reflects incomplete infection with the dominant negative virus coupled with incomplete blockade in the face of continued expression of endogenous NgR1.
As NgR1 intervention permits axonal sprouting and functional improvement long after injury, myelin inhibitors may be continuously suppressing CNS rewiring by stabilizing CNS anatomy. This function of myelin-associated inhibitors and NgR1 is consistent with the finding that NgR1 is required to restrict ocular dominance plasticity in the visual system to a developmental ‘critical period’. Adult NgR1 mutant mice preserve this experience-dependent plasticity at a level indistinguishable from adolescent ‘critical period’ mice66. Thus, myelin and NgR1 may contribute to consolidating neural circuitry and the efficacy of myelin neutralization to support functional improvement after chronic injury derives from the continuous physiologic role of myelin to dampen anatomical rearrangements at the synaptic level.
Acute blockade of NgR1 function within several days of spinal cord contusion improves locomotor recovery, with an average of a 2.75-point improvement on the BBB scale and two-fold increase in the occurrence of weight-bearing status67. The time dependence of benefits from NgR decoy treatment is difficult without direct comparison in the same experiment. However, the benefit for chronic injury appears of lesser magnitude than for treatment initiated within a week of contusion. This suggests that NgR1-independent factors allow the recently injured nervous system to more fully capitalize on a growth environment rendered more conducive to growth be NgR1 blockade.
The open field BMS and BBB scoring systems have played a key role in the analysis of motor dysfunction after spinal cord injury. Limb kinematic analysis is more detailed and provides additional insights into motor performance5, 59, 68, 69. While the use of these systems is increasing, much of the work has been restricted to body-weight supported treadmill stepping. Its use for spontaneous locomotion has been limited. Thus, baseline data for moderately injured mice and rats has few precedents in the literature. The strong correlation between recovery of raphespinal innervation in the lumbar spinal cord and kinematic parameters is consistent with fiber growth mediating return of function.
The type of movement impairment in mice with a chronic dorsal hemisection is distinct from rats with a moderate spinal contusion. Chronically-injured mice exhibited shortened swing of the foot relative to the hip. In agreement with previous short-term studies59, we observed a pronounced effect on limb position in chronically-injured rats. The foot was maintained in a posterior position despite relatively preserved anterior-posterior excursions with each gait cycle relative to the iliac crest. The use of such analysis in a broader range of studies is likely to provide greater insight into the mechanisms and pathways of recovery. In the current study, NgR1 intervention improved limb kinematics to more closely resemble the uninjured state despite the fact that treatment was initiated two months after injury.
Amongst the parameters derived from kinematic analysis are joint angles. However, the interpretation of such angles in cases of significant dysfunction without assisted stepping is not straightforward. We observed greater ankle angles through the gait cycle in mice with NgR1 deletion. This is consistent with greater strength and weight support, but it might reflect changes in posture or tone rather than strength or coordination. For the spinal contused rats, most animals were not weight-supporting and full ankle extension/flexion during sweeping gait cycles of the externally rotated hindlimb on the floor were noted. In the NgR-treated rats, ankle angles were linked with greater temporal precision to the gait cycle than in untreated rats. Moreover, the NgR-treated pattern more closely resembled the uninjured ankle angle pattern.
The translation of laboratory findings to clinical studies is strongly facilitated by quantitative biomarkers that monitor the primary mechanism of action. For spinal cord injury, functional analysis at the bedside using clinical scores has been the mainstay of analysis70. Electrophysiological analysis may provide additional confirmatory measurements, but these have seldom been used clinically or in injury models. To date, MR imaging studies have been used primarily to define the extent of injury rather than a degree of sprouting, plasticity, repair or recovery. Although the BOLD signal in functional MR studies and the diffusion tensor method may provide additional insights they cannot be considered a biomarker for the action of axon growth promoters71. Since our anatomical studies demonstrated highly branched sprouting of serotonergic fibers in the lumbar spinal cord, we sought a translatable method to monitor this growth. Positron emission tomography with serotonin transporter ligands provides a method to monitor the action of these interventions that drive neurological recovery and axon growth.
Our observation that chronically-injured animals can regain neurological function following administration of a single pharmacological reagent has two important implications. First, these findings demonstrate that at least one axon growth promoting therapeutic may have relevance for pre-existing spinal cord trauma. Second, these results may facilitate translational research because clinical trials of therapies for chronic spinal cord damage can be powered adequately with less than one-tenth the number of patients required for acute therapies72. In conclusion, perturbation of NgR1 pathways supports recovery of neurological function in chronic rodent spinal cord injury.
Supplementary Material
Acknowledgments
We thank Yiguang Fu and the Yale PET Center staff for outstanding technical assistance, and BiogenIdec Inc. for providing NgR-Fc fusion protein. A.W.M. holds a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund. This work was supported by the grants from the Christopher and Dana Reeve Foundation, the Wings for Life Foundation and the Dr. Ralph and Marion Falk Medical Research Trust to S.M.S., and from the National Institutes of Health to N.Y.H., L.I.B., W.B.J.C. and S.M.S.
Footnotes
Disclosure: S.M.S. is a co-founder of Axerion Therapeutics seeking to develop PrP and NgR therapies.
References
- 1.Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 5.Courtine G, Song B, Roy RR, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenzweig ES, Courtine G, Jindrich DL, et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cafferty WB, McGee AW, Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity? Trends Neurosci. 2008;31:215–220. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(NSCISC) NSCISC. SPINAL CORD INJURY, Facts and Figures at a Glance, June 2006. National Spinal Cord Injury Statistical Center (NSCISC); 2006. [Google Scholar]
- 10.Kadoya K, Tsukada S, Lu P, et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, et al. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tom VJ, Sandrow-Feinberg HR, Miller K, et al. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 14.Dimou L, Schnell L, Montani L, et al. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cafferty WB, Kim JE, Lee JK, Strittmatter SM. Response to correspondence: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B.” Neuron 38, 187–199. Neuron. 2007;54:195–199. doi: 10.1016/j.neuron.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JK, Geoffroy CG, Chan AF, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JE, Li S, GrandPre T, et al. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 19.Simonen M, Pedersen V, Weinmann O, et al. Systemic Deletion of the Myelin-Associated Outgrowth Inhibitor Nogo-A Improves Regenerative and Plastic Responses after Spinal Cord Injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng B, Atwal J, Ho C, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atwal JK, Pinkston-Gosse J, Syken J, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 24.Omoto S, Ueno M, Mochio S, et al. Genetic deletion of paired immunoglobulin-like receptor B does not promote axonal plasticity or functional recovery after traumatic brain injury. J Neurosci. 2010;30:13045–13052. doi: 10.1523/JNEUROSCI.3228-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura Y, Fujita Y, Ueno M, et al. Paired Immunoglobulin-like Receptor B Knockout Does Not Enhance Axonal Regeneration or Locomotor Recovery after Spinal Cord Injury. J Biol Chem. 2011;286:1876–1883. doi: 10.1074/jbc.M110.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebscher T, Schnell L, Schnell D, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 27.Wiessner C, Bareyre FM, Allegrini PR, et al. Anti-Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2003;23:154–165. doi: 10.1097/01.WCB.0000040400.30600.AF. [DOI] [PubMed] [Google Scholar]
- 28.Freund P, Schmidlin E, Wannier T, et al. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 29.Zorner B, Schwab ME. Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci. 2010;1198 (Suppl 1):E22–34. doi: 10.1111/j.1749-6632.2010.05566.x. [DOI] [PubMed] [Google Scholar]
- 30.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y, Shumsky JS, Sabol MA, et al. Nogo-66 receptor antagonist peptide (NEP1–40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabil Neural Repair. 2008;22:262–278. doi: 10.1177/1545968307308550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang PC, Barbay S, Plautz EJ, et al. Combination of NEP 1–40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544–549. doi: 10.1161/STROKEAHA.109.572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zai L, Ferrari C, Dice C, et al. Inosine augments the effects of a Nogo receptor blocker and of environmental enrichment to restore skilled forelimb use after stroke. Journal of Neuroscience. 2011 doi: 10.1523/JNEUROSCI.4498-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steward O, Sharp K, Yee KM, Hofstadter M. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp Neurol. 2008;209:446–468. doi: 10.1016/j.expneurol.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006 doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Liu BP, Budel S, et al. Blockade of nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvey PA, Lee DH, Qian F, et al. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J Neurosci. 2009;29:6285–6295. doi: 10.1523/JNEUROSCI.5885-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duffy P, Schmandke A, Sigworth J, et al. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci. 2009;29:15266–15276. doi: 10.1523/JNEUROSCI.4650-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dergham P, Ellezam B, Essagian C, et al. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann M, Fournier A, Selles-Navarro I, et al. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 46.Basso DM, Beattie MS, Bresnahan JC, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 47.Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- 48.Park JH, Gimbel DA, GrandPre T, et al. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci. 2006;26:1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinreb PH, Wen D, Qian F, et al. Resolution of disulfide heterogeneity in Nogo receptor I fusion proteins by molecular engineering. Biotechnol Appl Biochem. 2010;57:31–45. doi: 10.1042/BA20100061. [DOI] [PubMed] [Google Scholar]
- 50.Basso DM, Fisher LC, Anderson AJ, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 51.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 52.Cafferty WB, Yang SH, Duffy PJ, et al. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Kim JE, Budel S, et al. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams WA, Neumeister A, Nabulsi N, et al. First in human evaluation of [11C]AFM, a novel PET tracer for the serotonin transporter. NeuroImage. 2008;41:T42–T42. [Google Scholar]
- 55.Huang Y, Hwang DR, Bae SA, et al. A new positron emission tomography imaging agent for the serotonin transporter: synthesis, pharmacological characterization, and kinetic analysis of [11C]2-[2-(dimethylaminomethyl)phenylthio]-5-fluoromethylphenylamine ([11C]AFM) Nuclear medicine and biology. 2004;31:543–556. doi: 10.1016/j.nucmedbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, et al. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- 58.Berkelaar M, Clarke DB, Wang YC, et al. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collazos-Castro JE, Lopez-Dolado E, Nieto-Sampedro M. Locomotor deficits and adaptive mechanisms after thoracic spinal cord contusion in the adult rat. J Neurotrauma. 2006;23:1–17. doi: 10.1089/neu.2006.23.1. [DOI] [PubMed] [Google Scholar]
- 60.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 61.Liu K, Lu Y, Lee JK, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fry EJ, Chagnon MJ, Lopez-Vales R, et al. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58:423–433. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- 63.Shen Y, Tenney AP, Busch SA, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bareyre FM, Kerschensteiner M, Raineteau O, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Budel S, Baughman K, et al. Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J Neurotrauma. 2009;26:81–95. doi: 10.1089/neu.2007.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGee AW, Yang Y, Fischer QS, et al. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nessler JA, De Leon RD, Sharp K, et al. Robotic gait analysis of bipedal treadmill stepping by spinal contused rats: characterization of intrinsic recovery and comparison with BBB. J Neurotrauma. 2006;23:882–896. doi: 10.1089/neu.2006.23.882. [DOI] [PubMed] [Google Scholar]
- 69.Pereira JE, Cabrita AM, Filipe VM, et al. A comparison analysis of hindlimb kinematics during overground and treadmill locomotion in rats. Behav Brain Res. 2006;172:212–218. doi: 10.1016/j.bbr.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 70.Steeves JD, Lammertse D, Curt A, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- 71.Harel NY, Strittmatter SM. Functional MRI and other non-invasive imaging technologies: Providing visual biomarkers for spinal cord structure and function after injury. Exp Neurol. 2008;211:324–328. doi: 10.1016/j.expneurol.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.