Abstract
The presence of proenkephalin mRNA in germ cells purified from adult mouse testis were examined using RNA gel- and dot-blot analyses. Both pachytene spermatocytes and round spermatids were shown to contain concentrations of this transcript that are 2- to 3-fold greater than that found for whole mouse testis, on a per microgram of polyadenylylated RNA basis. The detection of proenkephalin mRNA in purified spermatocytes and spermatids could not be accounted for by contamination by either Leydig or Sertoli cells. No proenkephalin mRNA was detectable in extracts of mature sperm. These data suggest that developing germ cells may be a major site of proenkephalin synthesis in the adult testis and that proenkephalin-derived peptides may function as germ cell-associated hormones or autocrine/paracrine factors.
Full text
PDF

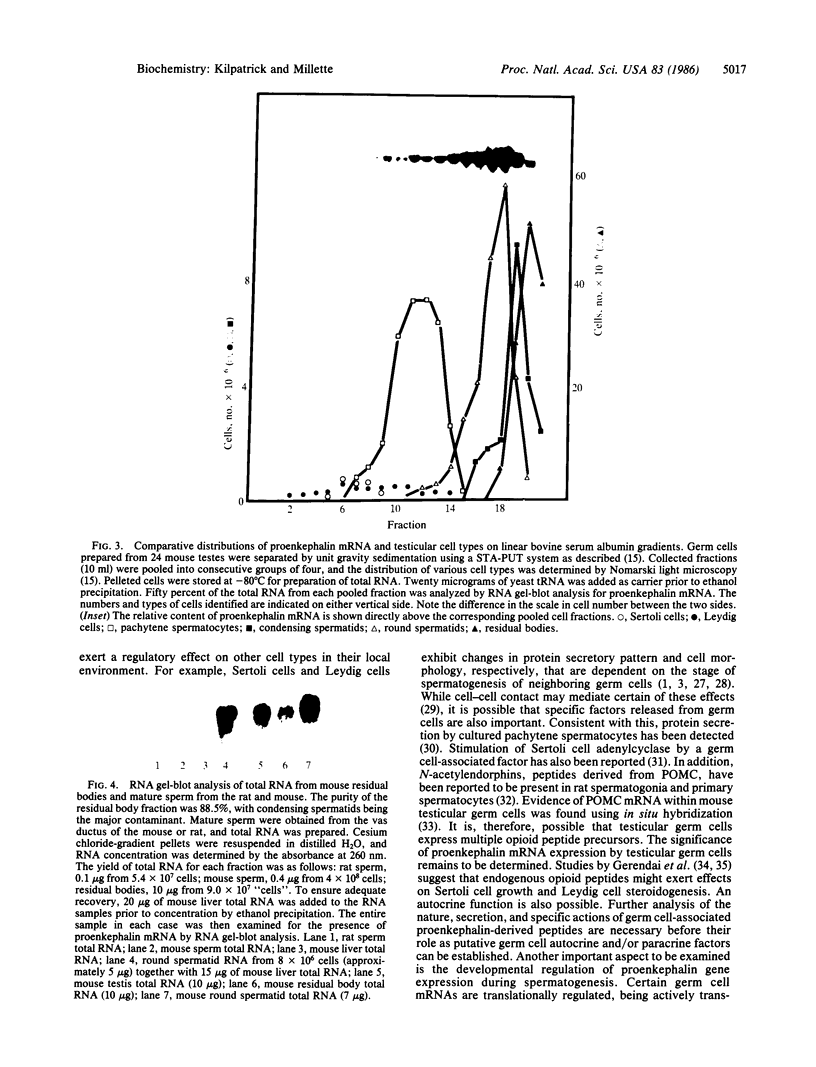

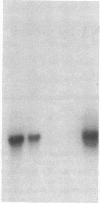
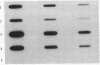
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bellvé A. R., Millette C. F., Bhatnagar Y. M., O'Brien D. A. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977 Jul;25(7):480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- Bergh A. Paracrine regulation of Leydig cells by the seminiferous tubules. Int J Androl. 1983 Feb;6(1):57–65. doi: 10.1111/j.1365-2605.1983.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Mather J. P., Morris P. L., Bardin C. W. Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5672–5675. doi: 10.1073/pnas.81.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. C., Clements J. A., Smith A. I., Lolait S. J., Funder J. W. N-acetyl endorphin in rat spermatogonia and primary spermatocytes. J Clin Invest. 1985 Mar;75(3):832–835. doi: 10.1172/JCI111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Civelli O., Douglass J., Goldstein A., Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. P., Erickson J. M., Betlach C. J., Meistrich M. L. Further evidence for haploid gene expression during spermatogenesis: heterogeneous, poly(A)- containing RNA is synthesized post-meiotically. J Exp Zool. 1980 Oct;214(1):13–19. doi: 10.1002/jez.1402140103. [DOI] [PubMed] [Google Scholar]
- Galdieri M., Monaco L. Evidence of protein secretion by cultured pachytene spermatocytes. Cell Differ. 1983 Sep;13(1):49–55. doi: 10.1016/0045-6039(83)90076-3. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Nemeskéri A., Csernus V. Naloxone has a local effect on the testis of immature rats. Andrologia. 1983 Jul-Aug;15(4):398–403. doi: 10.1111/j.1439-0272.1983.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Shaha C., Thau R., Bardin C. W. Do testicular opiates regulate Leydig cell function? Endocrinology. 1984 Oct;115(4):1645–1647. doi: 10.1210/endo-115-4-1645. [DOI] [PubMed] [Google Scholar]
- Gizang-Ginsberg E., Wolgemuth D. J. Localization of mRNAs in mouse testes by in situ hybridization: distribution of alpha-tubulin and developmental stage specificity of pro-opiomelanocortin transcripts. Dev Biol. 1985 Oct;111(2):293–305. doi: 10.1016/0012-1606(85)90484-1. [DOI] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bhatt R., Monahan J. J., Poonian M., Udenfriend S. Molecular cloning and sequence determination of rat preproenkephalin cDNA: sensitive probe for studying transcriptional changes in rat tissues. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7651–7655. doi: 10.1073/pnas.81.23.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Noe M., Bailey L. C., Udenfriend S. Expression of preproenkephalin-like mRNA and its peptide products in mammalian testis and ovary. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7467–7469. doi: 10.1073/pnas.82.21.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984 Sep;105(1):71–79. doi: 10.1016/0012-1606(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. cDNA clones encoding cytoplasmic poly(A)+ RNAs which first appear at detectable levels in haploid phases of spermatogenesis in the mouse. Dev Biol. 1983 Aug;98(2):455–464. doi: 10.1016/0012-1606(83)90375-5. [DOI] [PubMed] [Google Scholar]
- MONESI V. RIBONUCLEIC ACID SYNTHESIS DURING MITOSIS AND MEIOSIS IN THE MOUSE TESTIS. J Cell Biol. 1964 Sep;22:521–532. doi: 10.1083/jcb.22.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather J. P., Gunsalus G. L., Musto N. A., Cheng C. Y., Parvinen M., Wright W., Pérez-Infante V., Margioris A., Liotta A., Becker R. The hormonal and cellular control of Sertoli cell secretion. J Steroid Biochem. 1983 Jul;19(1A):41–51. [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Hirose T., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982 Jan 21;295(5846):202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Teranishi Y., Takahashi H., Toyosato M., Notake M., Nakanishi S., Numa S. Isolation and structural organization of the human preproenkephalin gene. Nature. 1982 Jun 3;297(5865):431–434. doi: 10.1038/297431a0. [DOI] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- ROOSEN-RUNGE E. C. Quantitative studies on spermatogenesis in the albino rat. III. Volume changes in the cells of the seminiferous tubules. Anat Rec. 1955 Dec;123(4):385–398. doi: 10.1002/ar.1091230402. [DOI] [PubMed] [Google Scholar]
- Ritzen E. M., Boitani C., Parvinen M., French F. C., Feldman M. Stage-dependent secretion of ABP by rat seminiferous tubules. Mol Cell Endocrinol. 1982 Jan;25(1):25–33. doi: 10.1016/0303-7207(82)90166-6. [DOI] [PubMed] [Google Scholar]
- Ritzén E. M. Chemical messengers between Sertoli cells and neighbouring cells. J Steroid Biochem. 1983 Jul;19(1B):499–504. doi: 10.1016/0022-4731(83)90209-1. [DOI] [PubMed] [Google Scholar]
- Romrell L. J., Bellvé A. R., Fawcett D. W. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976 Mar;49(1):119–131. doi: 10.1016/0012-1606(76)90262-1. [DOI] [PubMed] [Google Scholar]
- Russell L. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977 Mar;148(3):301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- Sastry B. V., Janson V. E., Owens L. K., Tayeb O. S. Enkephalin- and substance P-like immunoreactivities of mammalian sperm and accessory sex glands. Biochem Pharmacol. 1982 Nov 1;31(21):3519–3522. doi: 10.1016/0006-2952(82)90637-2. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M. Intratesticular factors controlling testicular function. Biol Reprod. 1984 Feb;30(1):29–49. doi: 10.1095/biolreprod30.1.29. [DOI] [PubMed] [Google Scholar]
- Shu-Dong T., Phillips D. M., Halmi N., Krieger D., Bardin C. W. Beta-endorphin is present in the male reproductive tract of five species. Biol Reprod. 1982 Oct;27(3):755–764. doi: 10.1095/biolreprod27.3.755. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Fritz I. B. Testicular peritubular cells secrete a protein under androgen control that modulates Sertoli cell functions. Proc Natl Acad Sci U S A. 1985 Jan;82(1):114–118. doi: 10.1073/pnas.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Hagopian S. S., Ishijima S. Changes in sperm plasma membrane lipid diffusibility after hyperactivation during in vitro capacitation in the mouse. J Cell Biol. 1986 Apr;102(4):1372–1377. doi: 10.1083/jcb.102.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]