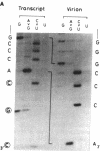
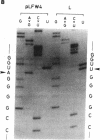
Abstract
We have cloned full-length double-stranded cDNAs of tobacco mosaic virus (TMV) (tomato strain L) RNA into a transcription vector, pPM1, which facilitates the correct transcription initiation from the first nucleotide of the inserted double-stranded cDNA, corresponding to the 5′ end of TMV RNA. When plasmid DNA is linearized at a unique restriction site (Mlu I) introduced just downstream of the double-stranded cDNA insert and used as a template for in vitro transcription by Escherichia coli RNA polymerase in the presence of m7GpppG, the transcribed RNAs are infectious for tobacco plants. A simple reconstitution procedure increases the infectivity >100 times. Unexpectedly, both the uncapped transcript and the transcript from the uncut plasmid DNA are also infectious, although their infectivities are very low. The progeny viruses multiplying in tobacco plants accurately reflect the cloned sequence. By the same method, we succeeded in the in vitro transcription of infectious RNA of attenuated strain L11A, which is phenotypically distinguishable from wild-type TMV on both tobacco and tomato plants.
Keywords: viral expression
Full text
PDF
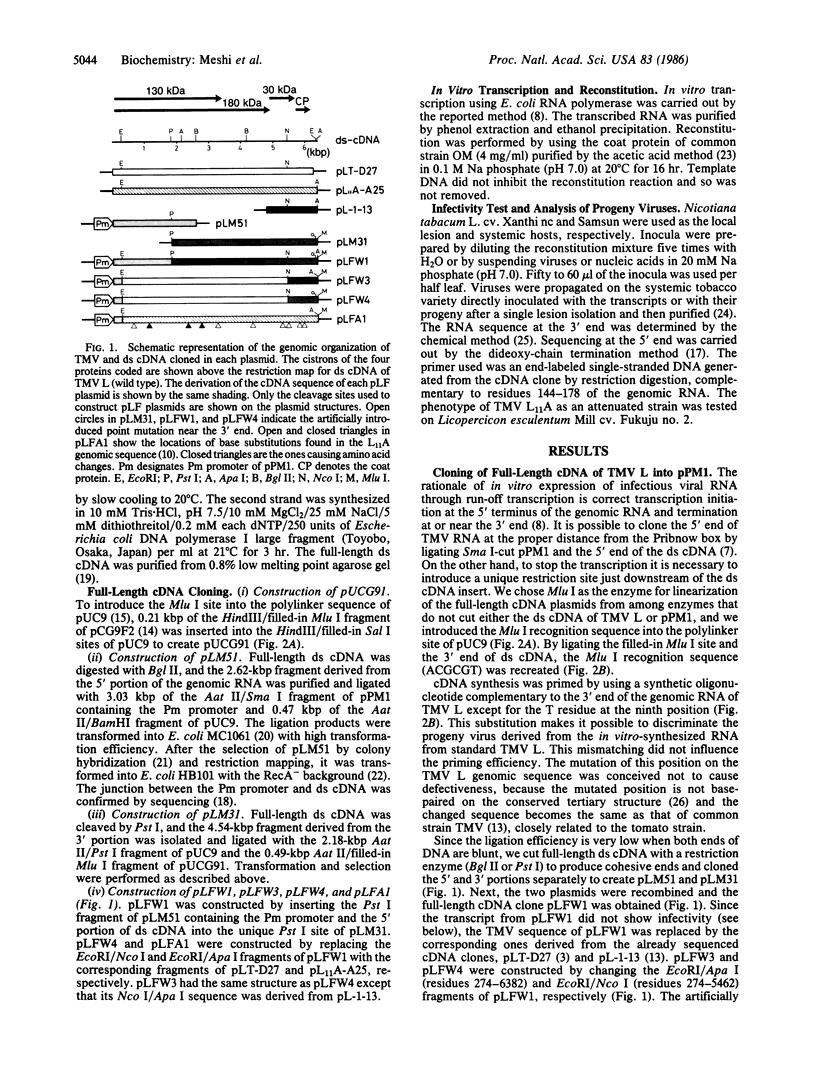
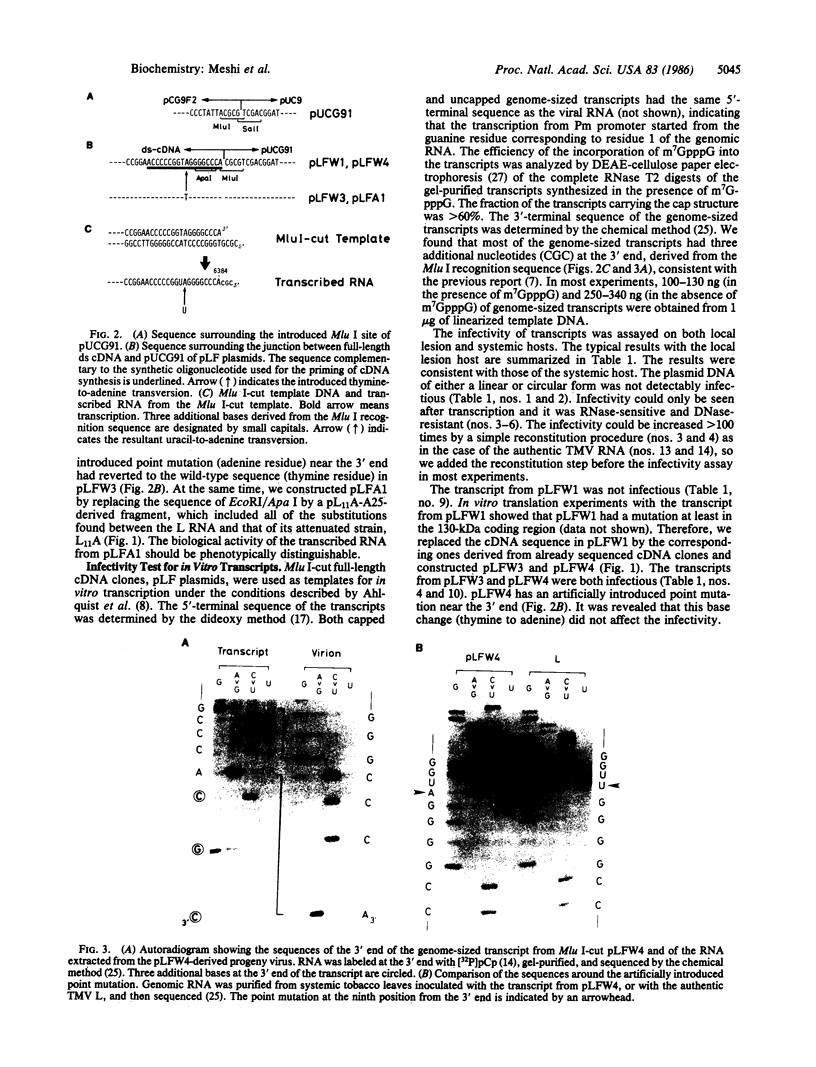


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Contreras R., Cheroutre H., Degrave W., Fiers W. Simple, efficient in vitro synthesis of capped RNA useful for direct expression of cloned eukaryotic genes. Nucleic Acids Res. 1982 Oct 25;10(20):6353–6362. doi: 10.1093/nar/10.20.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. Degradation of tobacco mosaic virus with acetic acid. Virology. 1957 Aug;4(1):1–4. doi: 10.1016/0042-6822(57)90038-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Ohno T., Okada Y., Otsuki Y., Takebe I. Kinetics of biphasic reconstitution of tobacco mosaic virus in vitro. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1727–1730. doi: 10.1073/pnas.75.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth L., Richards K. E. Tobacco mosaic virus: model for structure and function of a simple virus. Adv Virus Res. 1981;26:145–199. doi: 10.1016/s0065-3527(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. A revised replication cycle for viroids: the role of longer than unit length RNA in viroid replication. Mol Gen Genet. 1984;196(3):421–428. doi: 10.1007/BF00436189. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Takamatsu N., Ohno T., Okada Y. The 5'-terminal sequence of TMV RNA. Question on the polymorphism found in vulgare strain. FEBS Lett. 1983 Oct 17;162(2):282–285. doi: 10.1016/0014-5793(83)80772-8. [DOI] [PubMed] [Google Scholar]
- Nishiguchi M., Kikuchi S., Kiho Y., Ohno T., Meshi T., Okada Y. Molecular basis of plant viral virulence; the complete nucleotide sequence of an attenuated strain of tobacco mosaic virus. Nucleic Acids Res. 1985 Aug 12;13(15):5585–5590. doi: 10.1093/nar/13.15.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Aoyagi M., Yamanashi Y., Saito H., Ikawa S., Meshi T., Okada Y. Nucleotide sequence of the tobacco mosaic virus (tomato strain) genome and comparison with the common strain genome. J Biochem. 1984 Dec;96(6):1915–1923. doi: 10.1093/oxfordjournals.jbchem.a135026. [DOI] [PubMed] [Google Scholar]
- Ohno T., Okada Y., Shimotohno K., Miura K., Shinshi H. Enzymatic removal of the 5'-terminal methylated blocked structure of tobacco mosaic virus RNA and its effects on infectivity and reconstitution with coat protein. FEBS Lett. 1976 Aug 15;67(2):209–213. doi: 10.1016/0014-5793(76)80368-7. [DOI] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y., Nishiguchi M., Kiho Y. Single amino acid substitution in 30K protein of TMV defective in virus transport function. Virology. 1983 Nov;131(1):255–258. doi: 10.1016/0042-6822(83)90551-2. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld K., Linschooten K., Pleij C. W., Bosch L. The three-dimensional folding of the tRNA-like structure of tobacco mosaic virus RNA. A new building principle applied twice. EMBO J. 1984 Nov;3(11):2613–2619. doi: 10.1002/j.1460-2075.1984.tb02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Kodama Y., Hashimoto J., Miura K. I. Importance of 5'-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2734–2738. doi: 10.1073/pnas.74.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu N., Ohno T., Meshi T., Okada Y. Molecular cloning and nucleotide sequence of the 30K and the coat protein cistron of TMV (tomato strain) genome. Nucleic Acids Res. 1983 Jun 11;11(11):3767–3778. doi: 10.1093/nar/11.11.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zimmern D. The 5' end group of tobacco mosaic virus RNA is m7G5' ppp5' Gp. Nucleic Acids Res. 1975 Jul;2(7):1189–1201. doi: 10.1093/nar/2.7.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]