Abstract
Background
Coronary artery disease is the leading cause of death after transient ischemic attack (TIA). Reliable estimates of the risk of myocardial infarction (MI) after TIA, however, are lacking.
Purpose
To determine the incidence of and risk factors for MI after TIA
Methods
We cross-referenced pre-existing incidence cohorts from the Rochester Epidemiology Project for TIA (1985–1994) and MI (1979–2006) to identify all community residents with incident MI after incident TIA. Incidence of MI after TIA was determined using Kaplan-Meier life-table methods. This was compared to the age-, sex-, and period-specific MI incidence in the general population. Proportional hazards regression analysis was used to examine associations between clinical variables and the occurrence of MI after TIA.
Results
Average annual incidence of MI after TIA was 0.95%. Relative risk for incident MI in the TIA cohort compared to the general population was 2.09 (95% CI 1.52–2.81). This was highest in patients under 60 years old (relative risk 15.1; 95% CI 4.11–38.6). Increasing age (HR 1.51 per 10 years, 95% CI 1.14–2.01), male sex (HR 2.19, 95% CI 1.18–4.06), and the use of lipid lowering therapy at the time of TIA (HR 3.10, 95% CI 1.20–8.00) were independent risk factors for MI after TIA.
Conclusions
Average annual incidence of MI after TIA is approximately 1%, about double that of the general population. The relative risk increase is especially high in patients under 60 years old. These data are useful for identifying subgroups of patients with TIA at highest risk for subsequent MI.
Keywords: Transient Ischemic Attack, Myocardial Infarction, Incidence, Risk Factors, Mortality
Introduction
Cerebral ischemia and coronary artery disease (CAD) are epidemiologically and pathophysiologically closely related diseases 1. It logically follows and has been empirically demonstrated that they often coexist in the same patient. In fact, CAD has been shown to be the leading cause of death in patients with transient ischemic attacks (TIAs), causing more deaths than stroke. In five studies that have examined this topic, CAD has been responsible for 24% to 62% of deaths in the first 5 to 10 years after a TIA, while stroke accounted for 12% to 28%. In each study, fatal coronary events outnumbered fatal strokes by at least a factor of two.2–6
Despite the key role played by CAD in the mortality of patients with TIA, reliable estimates of the risk of MI after TIA and the excess risk of MI in those who have had a TIA compared with the general population are lacking.1 Although a number of existing studies have examined the risk of MI in patients with TIAs,3–5, 7–10 these studies have many important limitations. These include the use of inexact definitions for CAD-related outcomes and the absence of risk factor analysis for the prediction of MI after TIA. In addition, only one study investigating this topic includes patients whose index TIA occurred after the 1980’s.9 Only two of these studies have used a community-based approach, and neither of these used this design to estimate the difference in risk of MI between patients with TIA and the general population.7, 9
We sought to improve upon the existing knowledge about the risk of MI after TIA by performing a population-based study of events from a more recent time period using the resources of the Rochester Epidemiology Project.11 This method minimizes selection and ascertainment biases and allows for estimation of the magnitude of the excess risk of MI in TIA patients compared to the general population.11 Additionally, by using an extensive database of clinical variables defined using strict criteria and collected using a standardized protocol, we aimed to identify risk factors that mark those patients with TIA who are most likely to have an MI. Finally, we examined the role played by MI in the risk of death after TIA.
Methods
Study Cohort
We studied a community-based cohort of patients with incident TIA that was identified retrospectively through the Rochester Epidemiology Project Medical Records Linkage System. This system has been described thoroughly elsewhere.11 Rochester, Minnesota is located in Olmsted County, where virtually all medical care is provided by two institutions: Mayo Medical Center and Olmsted Medical Center, with their affiliated outpatient, emergency, and inpatient sites. The medical record data for all care provided for Olmsted County residents at these two institutions as well as at medical practices in the surrounding communities, University of Minnesota-affiliated hospitals, and the Veterans’ Administration Hospital in Minneapolis are recorded in a central computer index. This index is comprehensive, containing all inpatient and outpatient data, emergency department visits, nursing home care, and autopsy or death certificate information.
Using the Rochester Epidemiology Project, a cohort of Rochester residents who had incident TIAs between the years 1985 and 1994 and a cohort of Olmsted County residents who had incident MIs between 1979 and 2006 were identified. The procedures used to assemble these cohorts have been described in detail previously.12–14 Cases were initially identified from the medical record linkage system by a search using diagnostic codes. These diagnoses were then validated by chart review focusing on pre-specified criteria performed by physicians and nurse abstractors using standardized protocols. This method of case ascertainment has been shown to be robust, providing results similar to a cohort approach.15 Finally, extensive data regarding potential risk factors for the diseases in question were obtained using standardized protocols and risk factor definitions.
The definition of TIA used in this study is the same as that used in an earlier study of this cohort by Brown and colleagues.14 In brief, a TIA was defined as an episode of focal neurologic symptoms with abrupt onset and rapid resolution, lasting <24 hours and due to focal cerebral ischemia. Transient monocular blindness (TMB), which was included in the definition of TIA, was defined as an episode of transient monocular visual disturbance with abrupt onset and rapid resolution, lasting <24 hours, and due to retinal ischemia. The diagnosis of TIA was made by a single senior stroke neurologist (RDB) based on his review of detailed data from patients’ complete medical records. For any cases in which that senior stroke neurologist was uncertain of the diagnosis of TIA, the case was reviewed in detail by two other senior stroke neurologists. A final determination of TIA or no TIA was then made by consensus opinion of these three. TIA etiology was classified as large artery atherosclerosis, cardioembolic, lacunar, unknown, or other according to the classification scheme of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) 16. Although the TOAST classification system is most commonly used in categorizing ischemic strokes, the subtype definitions readily apply to patients with TIA. The exception is the lacunar subtype, given that infarct topography is of importance in its definition. For the TIA subtype categorization used in this study, patients with pure motor, pure sensory, or sensorimotor symptoms, which would otherwise fit the lacunar stroke criteria if they were longer in duration, were categorized as lacunar TIAs.
Ascertainment of MI relied on epidemiologic criteria combining cardiac pain, cardiac biomarkers, and electrocardiography. Details of this process, which has been shown to have excellent reliability, have been described in previous studies.13, 17 The ascertainment of MI events in the Olmsted County population achieved by using this process is complete, as evidenced by the fact that MI rates determined by it are commensurate with MI rates in the other surveillance programs in the United States.18, 19
We cross-referenced the TIA and MI cohorts to identify those Rochester residents who had an incident MI after incident TIA. In patients in whom incident MI pre-dated incident TIA, non-incident MIs were identified using a method identical to that used to create the incident MI cohort. Using these methods, we identified Rochester residents who had a MI through 2006 after an incident TIA that occurred during the years 1985 to 1994.
Statistical methods
The incidence of MI after TIA was determined using Kaplan-Meier life-table methods. This was compared to the age-, sex-, and period-specific MI incidence in the general population of Olmsted County using a one-sample log-rank test. The relative risk was calculated by dividing the number of observed MI’s by the expected number of MI’s over the duration of follow-up. Confidence intervals were calculated according to the Poisson distribution. We used proportional hazards regression analysis to examine associations between clinical variables and the occurrence of MI after TIA and to examine the association between MI and death after TIA. Sex as well as those variables associated with an altered hazard ratio with a p-value ≤ 0.15 in the univariate analysis were entered into the multivariable predictive models. MI was analyzed as a time-dependent covariate in the mortality model. The correlation of the scaled Schoenfeld residuals with time was used to test the proportional hazards assumption. All proportional hazards assumptions were met for the survival free of MI. Antithrombotic treatment was associated with a greater reduced risk of death in the first two years after TIA. Because the covariate only had a slight non-proportionality, and was not of primary interest, it was included as an adjustor in the mortality model. Analyses were done using the statistical software package SAS, version 8.2 (SAS Institute Inc., Cary, North Carolina).
Results
Cohort characteristics
There were 456 incident TIAs in Rochester residents between 1985 and 1994. Sixty-eight (15%) of these patients had a history of MI before TIA, of whom 8 (12%) had another MI after TIA. All subsequent results pertain to the 388 remaining patients who were at risk for incident MI after TIA. Follow-up data for this group was available for all but one patient through 5 years and was 98% and 72% complete at 10 and 20 years, respectively. Most patients with less than 20 years of follow-up were due to elapsed time limitations; only 15 patients were lost to follow-up (<4%) prior to the end of 2006 (the end of the MI data collection period).
Mean age at the time of TIA was 71±14 years and 41% of the patients were male (Table 1). Sixty-two percent had hypertension, 11% had diabetes mellitus, and 55% were current or former smokers. Based on TOAST subtypes, 19% of the TIAs were due to large artery atherosclerosis, 13% were cardioembolic, 6% were lacunar, and 5% were due to other causes. For 57% of the TIAs, a single etiology could not be clearly established.
Table 1.
Patient characteristics and univariate risk factor analysis for MI after TIA
| Variable | Total study population (n=456) | No MI before TIA (n=388) | No incident MI after TIA (n= 344) | Incident MI after TIA (n=44) | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Age at index TIA (mean ± SD; years) | 72 ± 14 | 71±14 | 71±15 | 72±12 | 1.42 (1.10–1.83)* | 0.007 |
| Male (%) | 43 | 41 | 40 | 55 | 1.53 (0.84–2.77) | 0.16 |
| Hypertension (%) | 64 | 62 | 61 | 70 | 2.02 (1.04–3.92) | 0.04 |
| Smoking (%) | 55 | 55 | 54 | 57 | 0.91 (0.50–1.66) | 0.75 |
| Diabetes (%) | 13 | 11 | 11 | 9 | 1.12 (0.40–3.14) | 0.83 |
| Lipid lowering treatment (%) | 5 | 5 | 4 | 11 | 2.42 (0.95–6.16) | 0.06 |
| Antithrombotic treatment (%) | 75 | 84 | 83 | 88 | 1.25 (0.49–3.18) | 0.64 |
| TIA etiology subtype (%) | ||||||
| Large artery atherosclerosis | 20 | 19 | 18 | 27 | 1.51 (0.78–2.93) | 0.22 |
| Cardioembolic | 15 | 13 | 14 | 9 | 0.74 (0.26–2.08) | 0.57 |
| Lacunar | 5 | 6 | 5 | 9 | 1.62 (0.58–4.54) | 0.36 |
| Unknown | 55 | 57 | 58 | 48 | 0.67 (0.37–1.22) | 0.19 |
| Other | 4 | 5 | 5 | 7 | 1.44 (0.45–4.68) | 0.54 |
Hazard Ratio is per 10 years
Abbreviations: MI: myocardial infarction; TIA: transient ischemic attack
Occurrence of MI after TIA
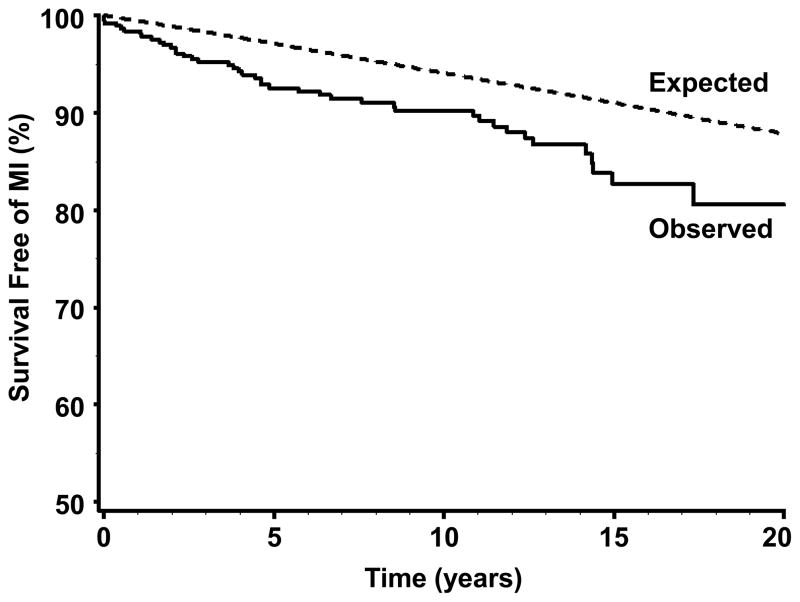
Median duration of follow-up for the 388 patients who were at risk for incident MI after TIA was 10.2 years (range 0 to 21.5 years). Forty-four of the patients in this group had an MI after TIA, yielding an average annual incidence of MI after TIA of 0.95%. Median time from index TIA to index MI was 4.6 years (range 0 to 20.6 years). Survival analysis shows that the risk of MI after TIA remained essentially constant over time (Figure). The age-, sex-, and period-specific relative risk (RR) for incident MI in patients who had a TIA, when compared to that of the general population, was 2.09 (95% CI 1.52–2.81). This elevated risk was most pronounced in TIA patients <60 years old, in whom the age-, sex-, and period-specific RR for MI was 15.1 (95% CI 4.11–38.6) when compared the general population. In comparison, for patients 60 years old and older, the age-, sex-, and period-specific RR for MI aggregated over three age groups (60–69, 70–79, and 80+ years old) was 1.93 (95% CI 1.41–2.63) when compared to the general population.
Figure.
Observed survival free of incident myocardial infarction after incident transient ischemic attack (solid line) and expected survival free of myocardial infarction in the general population of Olmsted County, Minnesota (dotted line).
Predictors of MI after TIA
In the univariate proportional hazards analysis, increasing age and hypertension were associated with an increased risk of incident MI after TIA (Table 1). Use of lipid lowering agents at the time of the TIA showed a trend toward association with an increased risk of MI after TIA (p=0.06). Multivariable analysis identified increasing age (HR 1.51 per 10 years, 95% CI 1.14–2.01), male sex (HR 2.19, 95% CI 1.18–4.06), and the use of lipid lowering therapy at the time of TIA (HR 3.10, 95% CI 1.20–8.00) to be independent risk factors for MI after TIA (Table 2). In this model, the association between hypertension and the risk of MI after TIA was no longer statistically significant (HR 1.76, 95% CI 0.90–3.45). TIA etiology was not a significant predictor of MI after TIA. This analysis was limited, however, by the fact that a single etiology could not be identified in many patients.
Table 2.
Multivariable risk factor model for MI after TIA
| Variable | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Age (per 10 years) | 1.51 (1.14–2.01) | 0.004 |
| Male | 2.19 (1.18–4.06) | 0.01 |
| Hypertension | 1.76 (0.90–3.45) | 0.10 |
| Lipid lowering therapy | 3.10 (1.20–8.00) | 0.02 |
Effect of MI on mortality after TIA
Univariate analysis showed that patients who had an MI after TIA were approximately three times more likely to die during follow-up than those who did not have an MI after TIA (HR 3.19, 95% CI 2.19–4.66). When adjusted for other factors found to be associated with mortality in this group (Table 3), MI remained a significant and substantial independent predictor of death after TIA (HR 3.11, 95% CI 2.11–4.57).
Table 3.
Multivariable model for risk of mortality after TIA
| Variable | Hazard Ratio (95% CI) | p-value |
|---|---|---|
| Age (per 10 years) | 2.30 (2.00–2.63) | <0.001 |
| Male sex | 1.05 (0.79–1.40) | 0.72 |
| Hypertension | 1.28 (0.97–1.68) | 0.08 |
| Smoking | 1.30 (0.98–1.73) | 0.07 |
| Diabetes | 1.54 (1.05–2.28) | 0.03 |
| Antithrombotic treatment | 0.67 (0.49–0.92) | 0.01 |
| Myocardial infarction after TIA | 3.11 (2.11–4.57) | <0.001 |
Discussion
CAD is the leading cause of death in patients who have had a TIA, and the two diseases have much in common epidemiologically and pathophysiologically. Nonetheless, reliable estimates of absolute risk of MI after TIA, the degree of excess risk for MI associated with TIA, and knowledge about the factors marking those TIA patients with an increased risk of subsequent MI have been lacking. This study addresses these shortcomings of the existing knowledge about the risk of MI after TIA by studying population-based cohorts.
Six main findings emerge from this study. First, the average annual incidence of MI after TIA was about 1%. Second, in agreement with a recently published meta-analysis of this topic,10 this risk accumulated linearly over at least 20 years after the index TIA and the average time between an index TIA and subsequent MI was approximately 5 years. Third, patients with TIA had twice the risk of having an MI as the general population. Fourth, the age-adjusted risk for MI is especially high in patients whose index TIA occurred before the age of 60. Fifth, male sex, increasing age, and the use of lipid lowering therapy were independent risk factors for MI after TIA, while hypertension was possibly a risk factor. Finally, of the variables we examined, MI was the strongest independent risk factor for death after TIA.
Existing studies have found annual rates of MI after TIA to be between 2.1% and 6%.5, 7–10, 20 Several factors may be responsible for the lower annual incidence in the present study. While we included patients whose index TIA occurred between 1985 and 1994, only one of the previous studies examining this question enrolled patients after the mid-1980’s.9 The lower rate of MI after TIA in the present study may at least in part be due to improvement in the prevention of MI that has taken place since that time.21–23 Data from a recently published meta-analysis provide strong and direct support for this argument.10 The population-based analysis used in the present study may also play a role in the lower rate of MI after TIA compared to other studies by the minimizing the selection bias in favor of sicker patients inherent in the prospective hospital-based cohort designs employed by most of the earlier studies.3–5, 8, 11 Finally, we studied only incident MI after TIA, a distinction not made in previous studies.
There are limited data available from previous studies regarding risk factors for MI after TIA. In univariate analysis, Muuronen and Kaste found that hypertension, diabetes, peripheral arterial disease, a history of heart disease, and carotid territory TIAs were associated with an increased risk of MI after TIA.4 Heyman and colleagues identified diabetes mellitus, prior MI, prior angina pectoris, electrocardiographic abnormalities, and the presence of ulcerated plaques or obstruction of more than 50% in at least one carotid artery on conventional angiography as risk factors for MI after TIA.8 Age and sex were not examined as risk factors and multivariate analysis was not performed in either study. Finally, in multivariate analysis of data from a prospective hospital cohort, risk factors for a coronary event after TIA were increasing age, male sex, a history of ischemic heart disease, and a combination of carotid and vertebrobasilar TIAs at presentation.20
In multivariate analysis, we identified male sex, increasing age, and the use of lipid-lowering therapy as independent risk factors for MI, while TIA etiologic subtype and diabetes were not. Our findings regarding the risk conferred by age and sex are consistent with the current understanding of CAD risk factors and pathogenesis and are in agreement with the only other study that has examined this relationship.20 Although hypertension is an important risk factor for CAD,24 its role as a risk factor for MI after TIA is unclear from our data. Two factors could explain this. First, effective treatment of hypertension in our population could have obscured a difference in MI risk conferred by the disease. Second, a larger number of outcomes than the 44 MIs encountered in our population may have been necessary to discern a statistically significant difference between those with and without hypertension. Similarly, despite the fact that both MI and large artery cerebral infarctions and TIAs are caused by atherosclerosis, we did not find an association between any etiologic cerebral ischemia subtype and subsequent MI. This may have been because of the small number of outcome events (MIs) in conjunction with relatively few patients within each etiologic subtype group. Finally, we found that the use of lipid-lowering therapy was an independent predictor of MI after TIA, perhaps because the use of such medications served as a surrogate marker for dyslipidemia and more severe atherosclerosis. Unfortunately, data regarding specific serum lipid measurements was relatively incomplete in our population for this issue to be meaningfully investigated more directly.
In the population of patients with TIA we studied, having a MI increased the risk of death by a factor of three. Given that MI has been shown by many earlier studies to be the major cause of death after TIA,2–6 this finding is not surprising. Nonetheless, it supports the concept of careful primary prevention of CAD in TIA patients, even in the absence of symptoms. It also suggests that screening for CAD in at least some TIA patients may be reasonable.
Strengths of this study include the population-based design, long and complete follow-up, standardized data collection techniques, and strict definitions for TIA, MI, and potential risk factors. Nonetheless, this study also has a number of limitations. First, racial and ethnic differences exist in the epidemiology of CAD25, 26 and ischemic cerebrovascular disease26, 27. As the population of Rochester is predominantly Caucasian, our findings might not be applicable to other racial and ethnic groups. Second, our community comparison population was matched for age, sex, and year of diagnosis but not for other variables that are known to affect the risk of MI. We were therefore not able to determine the amount of excess MI risk in our cohort caused by TIA alone. Third, insufficient data were available to optimally assess the effects of lipid and cholesterol disorders, key CAD risk factors, on the risk of MI after TIA. Fourth, the cohort size number of events may limit power to detect risk factors for MI after TIA. Fifth, as indicated by higher point estimates for the incidence of TIA in persons ≥85 year-old in the OXVASC data compared to our data, our case ascertainment for TIA may not have been complete in this age group.14, 28 This may have lead to under-representation of older people with TIAs in our analysis. However, the overall completeness of ascertainment of TIA cases in our data is supported by an earlier study that demonstrated the equivalence of cohort and medical records linkage methods in TIA case ascertainment15 as well as the comparable TIA incidence rates between the Rochester and Oxfordshire data for all other age groups. Finally, while more contemporary than that from most previously published studies, our study data are from a period that began over 20 years ago. Because the incidence of MI has diminished since then,21–23 our data may overestimate the risk of MI in present-day patients.
In conclusion, this study shows that patients who have had a TIA but do not have known CAD have approximately twice the risk for a subsequent MI compared to the general population. This risk accumulates linearly over time for many years after the TIA, with an incidence of TIA after MI of approximately 1% per year. Patients younger than 60 years old at the time of a TIA may have an especially high age-adjusted risk for future MI. Given that CAD plays an important role in the mortality of patients following TIA, these data support the existing concept1 that careful attention to the primary prevention of CAD is warranted in all patients who have had a TIA and that screening for asymptomatic CAD may be useful in select TIA patients.
Acknowledgments
Acknowledgements: none
Funding: Mayo Clinic
Footnotes
Conflicts of Interest/Disclosures:
Joseph D. Burns: Co-investigator in an investigator-initiated project with research funds provided by CardioNet.
Alejandro A Rabinstein: Principal investigator in an investigator-initiated project with research funds provided by CardioNet.
Veronique L. Roger: none
Latha G. Stead: none
Teresa J. H. Christianson, BS: none
Jill M. Killian: none
Robert D. Brown, Jr: none
Author Contributions:
Burns: study conception and design, data acquisition, manuscript drafting, critical review of manuscript
Rabinstein: study conception and design, study supervision, critical review of manuscript
Roger: study conception and design, data acquisition, study supervision, critical review of manuscript
Stead: critical review of manuscript
Christianson: data acquisition, statistical analysis, manuscript drafting, critical review of manuscript
Killian: data acquisition, statistical analysis
Brown: study conception and design, funding and supervision, manuscript drafting, critical review of manuscript
References
- 1.Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad PF, Taubert KA. Coronary risk evaluation in patients with transient ischemic attack and stroke: a scientific statement for healthcare professionals from the stroke council and the council on clinical cardiology of the American Heart Association/American Stroke Association. Stroke. 2003;34:2310–22. doi: 10.1161/01.STR.0000090125.28466.E2. [DOI] [PubMed] [Google Scholar]
- 2.Cartlidge NEF, Whisnant JP, Elveback LR. Carotid and vertebral-basilar transient ischemic attacks: a community study, Rochester, Minnesota. Mayo Clin Proc. 1977;52:117–20. [PubMed] [Google Scholar]
- 3.Hankey GJ, Slattery JM, Warlow CP. The prognosis of hospital-referred transient ischemic attacks. J Neurol Neurosurg Psychiatry. 1991;54:793–802. doi: 10.1136/jnnp.54.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muuronen A, Kaste M. Outcome of 314 patients with transient ischemic attack. Stroke. 1982;13:24–31. doi: 10.1161/01.str.13.1.24. [DOI] [PubMed] [Google Scholar]
- 5.Clark TG, Murphy MFG, Rothwell PM. Long term risks of stroke, myocardial infarction, and vascular death in “low risk” patients with a non-recent transient ischemic attack. J Neurol Neurosurg Psychiatry. 2003;74:577–80. doi: 10.1136/jnnp.74.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toole JF, Yuson CP, Janeway R, Johnston F, Davis C, Cordell AR, Howard G. Transient ischemic attacks: a prospective study of 225 patients. Neurology. 1978;28:746–53. doi: 10.1212/wnl.28.8.746. [DOI] [PubMed] [Google Scholar]
- 7.Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke. 1990;21:848–53. doi: 10.1161/01.str.21.6.848. [DOI] [PubMed] [Google Scholar]
- 8.Heyman A, Wilkinson WE, Hurwitz BJ, Haynes CS, Utley CM, Rosati RA, Burch JG, Gore TB. Risk of ischemic heart disease in patients with TIA. Neurology. 1984;34:626–30. doi: 10.1212/wnl.34.5.626. [DOI] [PubMed] [Google Scholar]
- 9.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population- based study. Neurology. 2004;62:2015–20. doi: 10.1212/01.wnl.0000129482.70315.2f. [DOI] [PubMed] [Google Scholar]
- 10.Touze E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas J-L. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke. Stroke. 2005;36:2748–55. doi: 10.1161/01.STR.0000190118.02275.33. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.Witt BJ, Brown RD, Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med. 2005;143:785–92. doi: 10.7326/0003-4819-143-11-200512060-00006. [DOI] [PubMed] [Google Scholar]
- 13.Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, Kottke TE, Yawn BP, Frye RL. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–8. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 14.Brown RD, Jr, Petty GW, O’Fallon WP, Wiebers DO, Whisnant JP. Incidence of transient ischemic attack in Rochester, Minnesota, 1985–1989. Stroke. 1998;29:2109–13. doi: 10.1161/01.str.29.10.2109. [DOI] [PubMed] [Google Scholar]
- 15.Whisnant JP, Melton LJ, Davis PF, O’Fallon WP, Nishimaru K, Schoenberg BS. Comparison of case ascertainment by medical record linkage and cohort follow-up to determine incidence rates for transient ischemic attacks and stroke. J Clin Epidemiol. 1990;43:791–7. doi: 10.1016/0895-4356(90)90239-l. [DOI] [PubMed] [Google Scholar]
- 16.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Joacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–9. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang C-H, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Eng J Med. 1998;339:861–7. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 19.Yeh RW, Sydney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Eng J Med. 2010;362:2155–65. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 20.Hankey GJ, Slattery JM, Warlow CP. Transient ischemic attacks: which patients are at high (and low) risk of serious vascular events? J Neurol Neurosurg Psychiatry. 1992;55:610–52. doi: 10.1136/jnnp.55.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arciero TJ, Jacobsen SJ, Reeder GS, Frye RL, Weston SA, Killian JM, Roger VL. Temporal trends in the incidence of coronary disease. Am J Med. 2004;117:228–33. doi: 10.1016/j.amjmed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Ergin A, Muntner P, Sherwin R, He J. Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med. 2004;117:219–27. doi: 10.1016/j.amjmed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA Project populations. Lancet. 1999;353:1547–57. doi: 10.1016/s0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- 24.Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr, Kaplan NM, O’Connor CM, O’Gara PT, Oparil S. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–88. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 25.Budoff MJ, Nasir K, Mao S, Tseng PH, Chau A, Liu ST, Flores F, Blumenthal RS. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis. 2006;187:343–50. doi: 10.1016/j.atherosclerosis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH. Cardiovascular disease and stroke in African-Americans and other racial minorities in the United States: a statement for health professionals. Introduction. Circulation. 1991;83:1463–5. doi: 10.1161/01.cir.83.4.1463. [DOI] [PubMed] [Google Scholar]
- 27.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. American Journal of Epidemiology. 1998;147:259–68. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 28.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JNE, Bull LM, Welch SJV, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–83. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]