Abstract
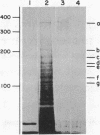
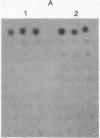
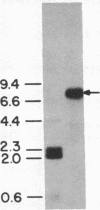
A chicken embryo sternal cartilage cDNA library, created in the plasmid expression vector pUC9, was screened for sequences coding for immunologically detectable core protein of the large, major proteoglycan of cartilage. A 1229-base-pair cDNA clone was isolated that contained only one extended open reading frame, which had sequences coding for a polypeptide of 379 amino acid residues. These deduced sequences corresponded to those anticipated from current models of proteoglycan structure; a deduced sequence encompassing 21 amino acids was almost identical to a known sequence of bovine nasal cartilage proteoglycan. Significant homology was found between the deduced amino acid sequence of the proteoglycan and two regions of a chicken hepatic lectin. Immunoprecipitation of the products of cell-free translation yielded a component of about 340 kDa, and transfer blot hybridization of sternal cartilage RNA showed a single mRNA of about 8.1 kilobases. Hybridizable mRNA sequences were readily detectable by dot-blot analyses of the cytoplasm of cartilaginous tissues of the chicken embryo, whereas similar analyses of prechondrogenic limb mesenchymal cells did not demonstrate such hybridizable mRNA signals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourdon M. A., Oldberg A., Pierschbacher M., Ruoslahti E. Molecular cloning and sequence analysis of a chondroitin sulfate proteoglycan cDNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1321–1325. doi: 10.1073/pnas.82.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter J. A., Rosenberg L. Structural changes during development in bovine fetal epiphyseal cartilage. Coll Relat Res. 1983 Nov;3(6):489–504. doi: 10.1016/s0174-173x(83)80028-4. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Dorfman A., Vertel B. M., Schwartz N. B. Immunological methods in the study of chondroitin sulfate proteoglycans. Curr Top Dev Biol. 1980;14(Pt 2):169–198. doi: 10.1016/s0070-2153(08)60194-5. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Complete amino acid sequence of a membrane receptor for glycoproteins. Sequence of the chicken hepatic lectin. J Biol Chem. 1981 Jun 10;256(11):5827–5839. [PubMed] [Google Scholar]
- Duchêne M., Sobel M. E., Müller P. K. Levels of collagen mRNA in dedifferentiating chondrocytes. Exp Cell Res. 1982 Dec;142(2):317–324. doi: 10.1016/0014-4827(82)90373-1. [DOI] [PubMed] [Google Scholar]
- Geetha-Habib M., Campbell S. C., Schwartz N. B. Subcellular localization of the synthesis and glycosylation of chondroitin sulfate proteoglycan core protein. J Biol Chem. 1984 Jun 10;259(11):7300–7310. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. H., Thonar E. J., Hascall V. C., Reiner A., Poole A. R. Identification of core protein, an intermediate in proteoglycan biosynthesis in cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Aug 10;256(15):7890–7897. [PubMed] [Google Scholar]
- Kosher R. A., Kulyk W. M., Gay S. W. Collagen gene expression during limb cartilage differentiation. J Cell Biol. 1986 Apr;102(4):1151–1156. doi: 10.1083/jcb.102.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- Mathews M. B. Comparative biochemistry of chondroitin sulphate-proteins of cartilage and notochord. Biochem J. 1971 Nov;125(1):37–46. doi: 10.1042/bj1250037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S. K., Kajiwara T., Ledger P. W., Tanzer M. L. Effects of the ionophore monensin on type II collagen and proteoglycan synthesis and secretion by cultured chondrocytes. J Biol Chem. 1982 Oct 10;257(19):11712–11716. [PubMed] [Google Scholar]
- Nishimoto S. K., Kajiwara T., Tanzer M. L. Proteoglycan core protein is accumulated in cultured chondrocytes in the presence of the ionophore monensin. J Biol Chem. 1982 Sep 25;257(18):10558–10561. [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Jollès J., Jollès P. Sequence data concerning the protein core of the cartilage proteoglycan monomers. Characterization of a sequence allowing the synthesis of an oligonucleotide probe. FEBS Lett. 1984 Oct 15;176(1):37–42. doi: 10.1016/0014-5793(84)80907-2. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikder S. K., Kabat E. A., Steer C. J., Ashwell G. The binding site of chicken hepatic lectin. J Biol Chem. 1983 Oct 25;258(20):12520–12525. [PubMed] [Google Scholar]
- Sparks K. J., Lever P. L., Goetinck P. F. Antibody binding of cartilage-specific proteoglycans. Arch Biochem Biophys. 1980 Feb;199(2):579–590. doi: 10.1016/0003-9861(80)90316-1. [DOI] [PubMed] [Google Scholar]
- Stevens J. W., Oike Y., Handley C., Hascall V. C., Hampton A., Caterson B. Characteristics of the core protein of the aggregating proteoglycan from the Swarm rat chondrosarcoma. J Cell Biochem. 1984;26(4):247–259. doi: 10.1002/jcb.240260405. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Vertel B. M., Dorfman A. Translation and characterization of messenger RNAs in differentiating chicken cartilage. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4847–4851. doi: 10.1073/pnas.76.10.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wiedemann H., Paulsson M., Timpl R., Engel J., Heinegård D. Domain structure of cartilage proteoglycans revealed by rotary shadowing of intact and fragmented molecules. Biochem J. 1984 Nov 15;224(1):331–333. doi: 10.1042/bj2240331. [DOI] [PMC free article] [PubMed] [Google Scholar]