Abstract
In the title compound, C14H12O5, the benzopyran-2-one ring system is approximately planar [maximum deviation = 0.018 (1) Å]; the mean plane is oriented at dihedral angles of 52.26 (11) and 72.92 (7)°, respectively, to the acetyl and acetoxy groups. In the crystal, π–π stacking is observed between parallel benzene rings of adjacent molecules, the centroid–centroid distance being 3.6774 (17) Å. Intermolecular weak C—H⋯O hydrogen bonding, and C=O⋯C=O [O⋯C = 3.058 (3) Å] and C=O⋯π [O⋯centroid = 3.328 (2) Å] interactions occur in the crystal structure.
Related literature
For structures of related coumarin derivatives, see: Yang et al. (2006 ▶, 2007 ▶, 2010 ▶).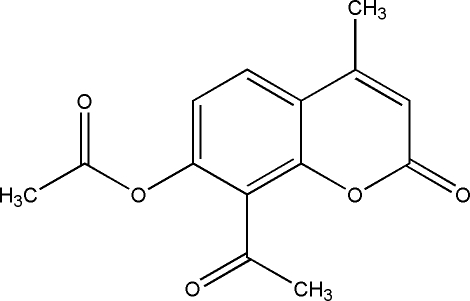
Experimental
Crystal data
C14H12O5
M r = 260.24
Triclinic,
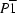
a = 8.198 (3) Å
b = 8.504 (3) Å
c = 9.644 (3) Å
α = 90.213 (4)°
β = 97.761 (4)°
γ = 111.686 (4)°
V = 618.0 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.11 mm−1
T = 298 K
0.30 × 0.10 × 0.10 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2001 ▶) T min = 0.969, T max = 0.989
4891 measured reflections
2292 independent reflections
1572 reflections with I > 2σ(I)
R int = 0.030
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.150
S = 1.07
2292 reflections
175 parameters
H-atom parameters constrained
Δρmax = 0.24 e Å−3
Δρmin = −0.19 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg & Berndt, 1999 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811048835/xu5385sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811048835/xu5385Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811048835/xu5385Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6—H6⋯O2i | 0.93 | 2.52 | 3.324 (3) | 145 |
Symmetry code: (i) 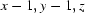 .
.
Acknowledgments
The project was supported by the Natural Science Foundation of Huaihai Institute of Technology, China (No. Z2009019)
supplementary crystallographic information
Comment
Previous we have reported the crystal structures of some coumarin derivatives (Yang et al., 2006, 2007, 2010). As part of our study of the crystal structures of coumarin derivatives, we decribed here the crystal structure of 8-acetyl-7-acetoxy-4-methyl-2H-1-benzopyran-2-one, (I).
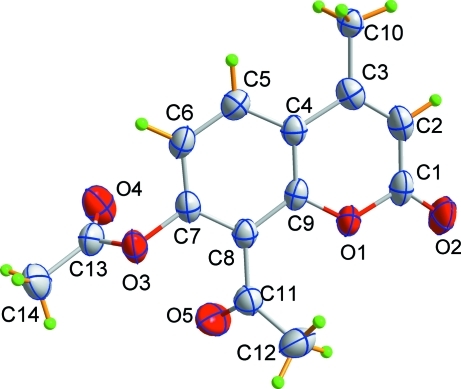
In the molecule (I) (Fig. 1), the benzopyran-2-one ring system is approximately palnar [maximum deviation 0.018 (1) Å]; the mean plane is oriented with respect to the acetyl and acetoxy groups at 52.26 (11) and 72.92 (7)°, respectively.
Molecules are linked together by one weak C—H···O hydrogen bond (Table. 1), two C═O···C═O interactions: C1═O2···C13ii═O4ii [O2···C13ii = 3.101 (3) Å, C1═O2···C13ii = 143.37 (15)°; symmetry code:(ii). x + 1, y + 1, z] and C1═O2···C1iii═ O2iii [O2···C1iii = 3.058 (3) Å, C1═O2···C1iii = 88.63 (13)°; symmetry code: (iii). 1 - x, 2 - y, 2 - z.], one C═ O···π interaction C1═O2···Cg1iii [O2···Cg1iii = 3.328 (2) Å, C1═O2···Cg1iii = 113.74 (14)°, Cg1 is the centroid of the pyran ring] and one π-π stacking interaction Cg2···Cg2iv [Cg2···Cg2iv = 3.6774 (17) Å, Cg2 is the centroid of the benzene ring; symmetry code: (iv) -x, 1 - y, 2 - z] and generated a two-dimensional crystal structure by translation and inversion symmetry.
Experimental
To a solution containing 8-acetyl-7-hydroxy- 4-methylcoumarin (2.18 g, 10 mmol) and anhydrous pyridine (10 ml), a solution of acetic anhydride (1.53 g, 15 mmol) was slowly added at 278–283 K, with stirring, then the reaction mixture was stirred at 303 K continuously for 24 h and then poured into ice–water (200 ml). The solid obtained was filtered off, washed with water and dried at room temperature. Colourless crystal suitable for X-ray structure analysis were obtained by recrystallizing the crude product from an ethanol solution [m.p. 484–485K].
Refinement
All H-atoms were placed in calculated positions (C—H = 0.93 and 0.96 Å) and were included in the refinement in the riding model, with Uiso(H) = 1.2Ueq(aromatic C) and Uiso(H) = 1.5Ueq(methyl C).
Figures
Fig. 1.
The molecular structure of the title compound, showing the atom-numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Crystal data
| C14H12O5 | Z = 2 |
| Mr = 260.24 | F(000) = 272 |
| Triclinic, P1 | Dx = 1.399 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.198 (3) Å | Cell parameters from 1283 reflections |
| b = 8.504 (3) Å | θ = 2.6–25.2° |
| c = 9.644 (3) Å | µ = 0.11 mm−1 |
| α = 90.213 (4)° | T = 298 K |
| β = 97.761 (4)° | Prism, colourless |
| γ = 111.686 (4)° | 0.30 × 0.10 × 0.10 mm |
| V = 618.0 (3) Å3 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 2292 independent reflections |
| Radiation source: fine-focus sealed tube | 1572 reflections with I > 2σ(I) |
| graphite | Rint = 0.030 |
| φ and ω scans | θmax = 25.5°, θmin = 2.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2001) | h = −9→9 |
| Tmin = 0.969, Tmax = 0.989 | k = −10→10 |
| 4891 measured reflections | l = −11→11 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.150 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0824P)2 + 0.0107P] where P = (Fo2 + 2Fc2)/3 |
| 2292 reflections | (Δ/σ)max < 0.001 |
| 175 parameters | Δρmax = 0.24 e Å−3 |
| 0 restraints | Δρmin = −0.19 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.3325 (3) | 1.0614 (2) | 0.9868 (2) | 0.0430 (5) | |
| C2 | 0.2915 (3) | 1.0571 (3) | 1.1282 (2) | 0.0463 (5) | |
| H2 | 0.3657 | 1.1427 | 1.1938 | 0.056* | |
| C3 | 0.1515 (3) | 0.9353 (2) | 1.1690 (2) | 0.0409 (5) | |
| C4 | 0.0343 (2) | 0.8014 (2) | 1.0672 (2) | 0.0354 (5) | |
| C5 | −0.1181 (3) | 0.6703 (2) | 1.0954 (2) | 0.0408 (5) | |
| H5 | −0.1486 | 0.6655 | 1.1852 | 0.049* | |
| C6 | −0.2241 (3) | 0.5482 (2) | 0.9946 (2) | 0.0419 (5) | |
| H6 | −0.3251 | 0.4615 | 1.0156 | 0.050* | |
| C7 | −0.1786 (2) | 0.5559 (2) | 0.8607 (2) | 0.0368 (5) | |
| C8 | −0.0288 (2) | 0.6828 (2) | 0.8251 (2) | 0.0349 (5) | |
| C9 | 0.0747 (2) | 0.8046 (2) | 0.9306 (2) | 0.0344 (5) | |
| C10 | 0.1127 (3) | 0.9338 (3) | 1.3169 (2) | 0.0646 (7) | |
| H10A | 0.2010 | 1.0299 | 1.3709 | 0.097* | |
| H10B | 0.1145 | 0.8314 | 1.3574 | 0.097* | |
| H10C | −0.0023 | 0.9390 | 1.3168 | 0.097* | |
| C11 | 0.0155 (3) | 0.6857 (2) | 0.6786 (2) | 0.0432 (5) | |
| C12 | 0.1973 (3) | 0.6967 (3) | 0.6594 (3) | 0.0593 (7) | |
| H12A | 0.1881 | 0.6088 | 0.5925 | 0.089* | |
| H12B | 0.2594 | 0.6830 | 0.7475 | 0.089* | |
| H12C | 0.2612 | 0.8052 | 0.6259 | 0.089* | |
| C13 | −0.4432 (3) | 0.4153 (3) | 0.7065 (2) | 0.0463 (5) | |
| C14 | −0.5350 (3) | 0.2647 (3) | 0.6076 (3) | 0.0634 (7) | |
| H14A | −0.6577 | 0.2494 | 0.5840 | 0.095* | |
| H14B | −0.5263 | 0.1661 | 0.6508 | 0.095* | |
| H14C | −0.4803 | 0.2813 | 0.5241 | 0.095* | |
| O1 | 0.21819 (16) | 0.93313 (16) | 0.89181 (14) | 0.0416 (4) | |
| O2 | 0.4560 (2) | 1.16541 (18) | 0.94235 (18) | 0.0601 (5) | |
| O3 | −0.27782 (17) | 0.42394 (16) | 0.76069 (15) | 0.0447 (4) | |
| O4 | −0.5014 (2) | 0.5178 (2) | 0.73698 (19) | 0.0684 (5) | |
| O5 | −0.0952 (2) | 0.6742 (2) | 0.57939 (17) | 0.0694 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0312 (11) | 0.0310 (11) | 0.0618 (14) | 0.0074 (9) | 0.0020 (10) | 0.0004 (10) |
| C2 | 0.0374 (12) | 0.0396 (11) | 0.0551 (14) | 0.0110 (9) | −0.0060 (10) | −0.0082 (10) |
| C3 | 0.0402 (11) | 0.0389 (11) | 0.0420 (12) | 0.0154 (9) | −0.0016 (9) | −0.0018 (9) |
| C4 | 0.0314 (10) | 0.0343 (10) | 0.0405 (11) | 0.0132 (8) | 0.0027 (8) | 0.0019 (8) |
| C5 | 0.0393 (11) | 0.0409 (11) | 0.0417 (11) | 0.0134 (9) | 0.0093 (9) | 0.0056 (9) |
| C6 | 0.0312 (10) | 0.0360 (11) | 0.0529 (13) | 0.0047 (8) | 0.0103 (9) | 0.0043 (9) |
| C7 | 0.0292 (10) | 0.0314 (10) | 0.0463 (12) | 0.0091 (8) | 0.0000 (8) | −0.0022 (8) |
| C8 | 0.0296 (10) | 0.0331 (10) | 0.0421 (11) | 0.0125 (8) | 0.0034 (8) | 0.0007 (8) |
| C9 | 0.0248 (9) | 0.0308 (10) | 0.0463 (12) | 0.0090 (8) | 0.0046 (8) | 0.0050 (8) |
| C10 | 0.0727 (17) | 0.0653 (16) | 0.0455 (14) | 0.0164 (13) | 0.0016 (12) | −0.0083 (12) |
| C11 | 0.0415 (12) | 0.0403 (11) | 0.0456 (12) | 0.0133 (9) | 0.0050 (10) | −0.0001 (9) |
| C12 | 0.0539 (14) | 0.0754 (16) | 0.0571 (15) | 0.0293 (12) | 0.0221 (12) | 0.0068 (12) |
| C13 | 0.0319 (11) | 0.0500 (12) | 0.0508 (13) | 0.0092 (10) | 0.0026 (9) | −0.0017 (10) |
| C14 | 0.0442 (14) | 0.0689 (16) | 0.0619 (16) | 0.0076 (12) | −0.0036 (12) | −0.0196 (13) |
| O1 | 0.0315 (7) | 0.0372 (8) | 0.0489 (9) | 0.0040 (6) | 0.0070 (6) | 0.0021 (6) |
| O2 | 0.0442 (9) | 0.0414 (9) | 0.0804 (12) | −0.0021 (7) | 0.0141 (8) | 0.0035 (8) |
| O3 | 0.0315 (8) | 0.0380 (8) | 0.0578 (9) | 0.0075 (6) | 0.0004 (6) | −0.0097 (7) |
| O4 | 0.0503 (10) | 0.0742 (12) | 0.0835 (13) | 0.0333 (9) | −0.0093 (9) | −0.0180 (10) |
| O5 | 0.0583 (11) | 0.1014 (14) | 0.0466 (10) | 0.0311 (10) | −0.0026 (8) | −0.0036 (9) |
Geometric parameters (Å, °)
| C1—O2 | 1.204 (2) | C9—O1 | 1.374 (2) |
| C1—O1 | 1.383 (2) | C10—H10A | 0.9600 |
| C1—C2 | 1.445 (3) | C10—H10B | 0.9600 |
| C2—C3 | 1.338 (3) | C10—H10C | 0.9600 |
| C2—H2 | 0.9300 | C11—O5 | 1.203 (2) |
| C3—C4 | 1.453 (3) | C11—C12 | 1.495 (3) |
| C3—C10 | 1.502 (3) | C12—H12A | 0.9600 |
| C4—C5 | 1.394 (3) | C12—H12B | 0.9600 |
| C4—C9 | 1.400 (3) | C12—H12C | 0.9600 |
| C5—C6 | 1.369 (3) | C13—O4 | 1.191 (2) |
| C5—H5 | 0.9300 | C13—O3 | 1.361 (2) |
| C6—C7 | 1.388 (3) | C13—C14 | 1.483 (3) |
| C6—H6 | 0.9300 | C14—H14A | 0.9600 |
| C7—C8 | 1.388 (3) | C14—H14B | 0.9600 |
| C7—O3 | 1.399 (2) | C14—H14C | 0.9600 |
| C8—C9 | 1.392 (3) | O2—C1i | 3.058 (3) |
| C8—C11 | 1.504 (3) | O2—C13ii | 3.101 (3) |
| O2—C1—O1 | 116.15 (19) | C3—C10—H10B | 109.5 |
| O2—C1—C2 | 126.87 (19) | H10A—C10—H10B | 109.5 |
| O1—C1—C2 | 116.97 (17) | C3—C10—H10C | 109.5 |
| C3—C2—C1 | 122.88 (19) | H10A—C10—H10C | 109.5 |
| C3—C2—H2 | 118.6 | H10B—C10—H10C | 109.5 |
| C1—C2—H2 | 118.6 | O5—C11—C12 | 121.1 (2) |
| C2—C3—C4 | 118.98 (19) | O5—C11—C8 | 120.33 (19) |
| C2—C3—C10 | 121.69 (19) | C12—C11—C8 | 118.50 (18) |
| C4—C3—C10 | 119.33 (19) | C11—C12—H12A | 109.5 |
| C5—C4—C9 | 117.28 (18) | C11—C12—H12B | 109.5 |
| C5—C4—C3 | 124.53 (18) | H12A—C12—H12B | 109.5 |
| C9—C4—C3 | 118.19 (18) | C11—C12—H12C | 109.5 |
| C6—C5—C4 | 121.83 (19) | H12A—C12—H12C | 109.5 |
| C6—C5—H5 | 119.1 | H12B—C12—H12C | 109.5 |
| C4—C5—H5 | 119.1 | O4—C13—O3 | 122.68 (19) |
| C5—C6—C7 | 118.96 (18) | O4—C13—C14 | 126.4 (2) |
| C5—C6—H6 | 120.5 | O3—C13—C14 | 110.89 (18) |
| C7—C6—H6 | 120.5 | C13—C14—H14A | 109.5 |
| C6—C7—C8 | 122.35 (18) | C13—C14—H14B | 109.5 |
| C6—C7—O3 | 119.13 (17) | H14A—C14—H14B | 109.5 |
| C8—C7—O3 | 118.30 (17) | C13—C14—H14C | 109.5 |
| C7—C8—C9 | 116.78 (18) | H14A—C14—H14C | 109.5 |
| C7—C8—C11 | 120.47 (17) | H14B—C14—H14C | 109.5 |
| C9—C8—C11 | 122.75 (17) | C9—O1—C1 | 121.70 (15) |
| O1—C9—C8 | 115.92 (17) | C1—O2—C1i | 88.63 (13) |
| O1—C9—C4 | 121.25 (17) | C1—O2—C13ii | 143.37 (15) |
| C8—C9—C4 | 122.81 (18) | C1i—O2—C13ii | 118.73 (8) |
| C3—C10—H10A | 109.5 | C13—O3—C7 | 117.16 (14) |
| O2—C1—C2—C3 | −179.5 (2) | C3—C4—C9—O1 | −1.9 (3) |
| O1—C1—C2—C3 | 0.7 (3) | C5—C4—C9—C8 | −0.8 (3) |
| C1—C2—C3—C4 | −0.3 (3) | C3—C4—C9—C8 | −179.87 (16) |
| C1—C2—C3—C10 | 179.75 (19) | C7—C8—C11—O5 | 50.4 (3) |
| C2—C3—C4—C5 | −178.21 (18) | C9—C8—C11—O5 | −129.3 (2) |
| C10—C3—C4—C5 | 1.7 (3) | C7—C8—C11—C12 | −127.4 (2) |
| C2—C3—C4—C9 | 0.8 (3) | C9—C8—C11—C12 | 52.9 (3) |
| C10—C3—C4—C9 | −179.22 (18) | C8—C9—O1—C1 | −179.47 (15) |
| C9—C4—C5—C6 | 0.5 (3) | C4—C9—O1—C1 | 2.4 (3) |
| C3—C4—C5—C6 | 179.52 (17) | O2—C1—O1—C9 | 178.42 (16) |
| C4—C5—C6—C7 | −0.2 (3) | C2—C1—O1—C9 | −1.7 (3) |
| C5—C6—C7—C8 | 0.2 (3) | O1—C1—O2—C1i | −87.95 (16) |
| C5—C6—C7—O3 | 174.65 (16) | C2—C1—O2—C1i | 92.2 (2) |
| C6—C7—C8—C9 | −0.4 (3) | O1—C1—O2—C13ii | 52.7 (3) |
| O3—C7—C8—C9 | −174.95 (15) | C2—C1—O2—C13ii | −127.1 (2) |
| C6—C7—C8—C11 | 179.84 (18) | O4—C13—O3—C7 | 2.9 (3) |
| O3—C7—C8—C11 | 5.3 (3) | C14—C13—O3—C7 | −177.22 (18) |
| C7—C8—C9—O1 | −177.37 (14) | C6—C7—O3—C13 | 73.3 (2) |
| C11—C8—C9—O1 | 2.3 (3) | C8—C7—O3—C13 | −112.06 (19) |
| C7—C8—C9—C4 | 0.7 (3) | C1—O2—C13ii—O4ii | 69.0 (3) |
| C11—C8—C9—C4 | −179.55 (17) | C1—O2—C1i—O2i | 0.0 |
| C5—C4—C9—O1 | 177.26 (15) |
Symmetry codes: (i) −x+1, −y+2, −z+2; (ii) x+1, y+1, z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6···O2iii | 0.93 | 2.52 | 3.324 (3) | 145. |
Symmetry codes: (iii) x−1, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU5385).
References
- Brandenburg, K. & Berndt, M. (1999). DIAMOND Crystal Impact GbR, Bonn, Germany
- Bruker (2001). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Yang, S.-P., Han, L.-J., Wang, D.-Q. & Chen, X.-Y. (2010). Acta Cryst. E66, o3183. [DOI] [PMC free article] [PubMed]
- Yang, S.-P., Han, L.-J., Wang, D.-Q. & Xia, H.-T. (2007). Acta Cryst. E63, o4643.
- Yang, S.-P., Han, L.-J., Xia, H.-T. & Wang, D.-Q. (2006). Acta Cryst. E62, o4181–o4182.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811048835/xu5385sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811048835/xu5385Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811048835/xu5385Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report