Abstract
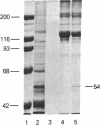
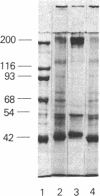
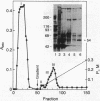
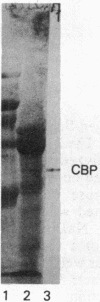
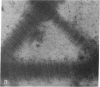
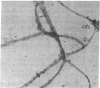
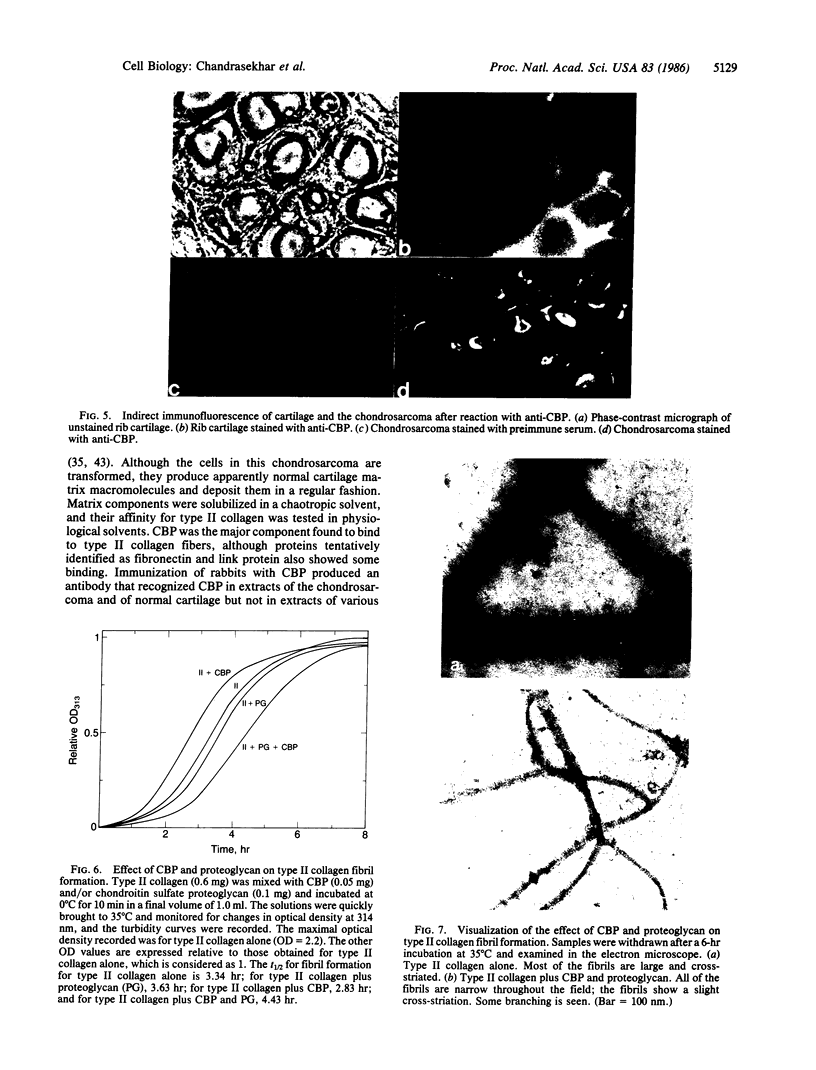
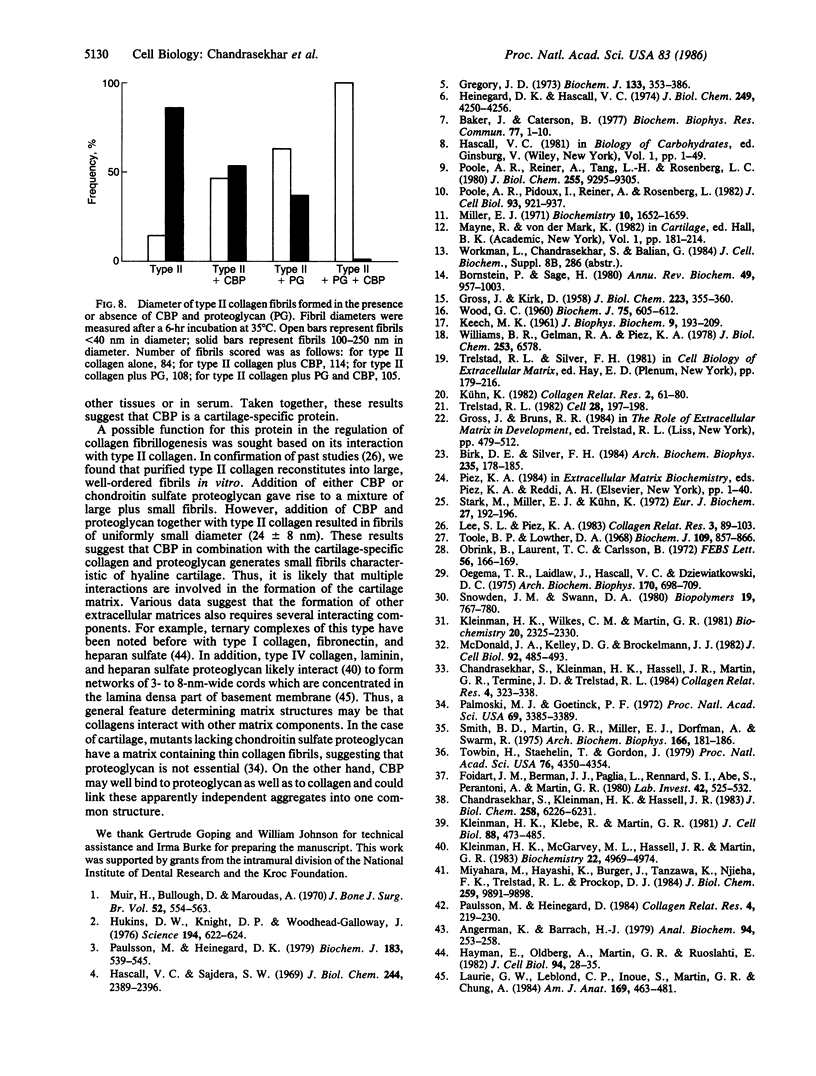
In cartilage, type II collagen is present as thin, short, randomly oriented fibrils. In vitro, however, type II collagen forms fibrils of large diameter, indicating that additional factors may be involved in the regulation of collagen fibril formation. We have examined extracts of a cartilage-producing tumor for the presence of collagen-binding proteins. In addition to fibronectin and link protein, a Mr 54,000 protein was found to bind to collagen fibrils as well as to native and denatured type II collagen. Immunological studies using antibody against the protein indicate that it is a cartilage matrix protein, not present in bone or in several other tissues. In vitro studies show that the Mr 54,000 protein in combination with cartilage proteoglycan decreases the rate of type II fibril formation and causes the fibrils to be of small diameter (24 +/- 8 nm). These studies indicate that complexes between collagen and proteoglycans mediated by this protein may regulate the assembly of cartilage matrix.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angermann K., Barrach H. J. Purification of procollagen type II by covalent chromatography with activated thiol-sepharose 4B. Anal Biochem. 1979 Apr 15;94(2):253–258. doi: 10.1016/0003-2697(79)90356-7. [DOI] [PubMed] [Google Scholar]
- Baker J., Caterson B. The purification and cyanogen bromide cleavage of the 'link proteins' from cartilage proteoglycan. Biochem Biophys Res Commun. 1977 Jul 11;77(1):1–10. doi: 10.1016/s0006-291x(77)80157-5. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Silver F. H. Collagen fibrillogenesis in vitro: comparison of types I, II, and III. Arch Biochem Biophys. 1984 Nov 15;235(1):178–185. doi: 10.1016/0003-9861(84)90266-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Kleinman H. K., Hassell J. R. Interaction of link protein with collagen. J Biol Chem. 1983 May 25;258(10):6226–6231. [PubMed] [Google Scholar]
- Chandrasekhar S., Kleinman H. K., Hassell J. R., Martin G. R., Termine J. D., Trelstad R. L. Regulation of type I collagen fibril assembly by link protein and proteoglycans. Coll Relat Res. 1984 Oct;4(5):323–337. doi: 10.1016/s0174-173x(84)80001-1. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Berman J. J., Paglia L., Rennard S., Abe S., Perantoni A., Martin G. R. Synthesis of fibronectin, laminin, and several collagens by a liver-derived epithelial line. Lab Invest. 1980 May;42(5):525–532. [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Gregory J. D. Multiple aggregation factors in cartilage proteoglycan. Biochem J. 1973 Jun;133(2):383–386. doi: 10.1042/bj1330383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hayman E. G., Oldberg A., Martin G. R., Ruoslahti E. Codistribution of heparan sulfate proteoglycan, laminin, and fibronectin in the extracellular matrix of normal rat kidney cells and their coordinate absence in transformed cells. J Cell Biol. 1982 Jul;94(1):28–35. doi: 10.1083/jcb.94.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Hukins D. W., Knight D. P., Woodhead-Galloway J. Amianthoid change: orientation of normal collagen fibrils during aging. Science. 1976 Nov 5;194(4265):622–624. doi: 10.1126/science.982030. [DOI] [PubMed] [Google Scholar]
- KEECH M. K. The formation of fibrils from collagen solutions. IV. Effect of mucopolysaccharides and nucleic acids: an electron microscope study. J Biophys Biochem Cytol. 1961 Jan;9:193–209. doi: 10.1083/jcb.9.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Martin G. R. Formation of a supramolecular complex is involved in the reconstitution of basement membrane components. Biochemistry. 1983 Oct 11;22(21):4969–4974. doi: 10.1021/bi00290a014. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Wilkes C. M., Martin G. R. Interaction of fibronectin with collagen fibrils. Biochemistry. 1981 Apr 14;20(8):2325–2330. doi: 10.1021/bi00511a039. [DOI] [PubMed] [Google Scholar]
- Kühn K. Segment-long-spacing crystallites, a powerful tool in collagen research. Coll Relat Res. 1982 Jan;2(1):61–80. doi: 10.1016/s0174-173x(82)80041-1. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Inoue S., Martin G. R., Chung A. Fine structure of the glomerular basement membrane and immunolocalization of five basement membrane components to the lamina densa (basal lamina) and its extensions in both glomeruli and tubules of the rat kidney. Am J Anat. 1984 Apr;169(4):463–481. doi: 10.1002/aja.1001690408. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Piez K. A. Type II collagen from lathyritic rat chondrosarcoma: preparation and in vitro fibril formation. Coll Relat Res. 1983 Mar;3(2):89–103. doi: 10.1016/s0174-173x(83)80036-3. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G., Broekelmann T. J. Role of fibronectin in collagen deposition: Fab' to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol. 1982 Feb;92(2):485–492. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Hayashi K., Berger J., Tanzawa K., Njieha F. K., Trelstad R. L., Prockop D. J. Formation of collagen fibrils by enzymic cleavage of precursors of type I collagen in vitro. J Biol Chem. 1984 Aug 10;259(15):9891–9898. [PubMed] [Google Scholar]
- Muir H., Bullough P., Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br. 1970 Aug;52(3):554–563. [PubMed] [Google Scholar]
- Obrink B., Laurent T. C., Carlsson B. The binding of chondroitin sulphate to collagen. FEBS Lett. 1975 Aug 1;56(1):166–169. doi: 10.1016/0014-5793(75)80133-5. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Laidlaw J., Hascall V. C., Dziewiatkowski D. D. The effect of proteoglycans on the formation of fibrils from collagen solutions. Arch Biochem Biophys. 1975 Oct;170(2):698–709. doi: 10.1016/0003-9861(75)90167-8. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Goetinck P. F. Synthesis of proteochondroitin sulfate by normal, nanomelic, and 5-bromodeoxyuridine-treated chondrocytes in cell culture. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3385–3388. doi: 10.1073/pnas.69.11.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Matrix proteins bound to associatively prepared proteoglycans from bovine cartilage. Biochem J. 1979 Dec 1;183(3):539–545. doi: 10.1042/bj1830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Noncollagenous cartilage proteins current status of an emerging research field. Coll Relat Res. 1984 May;4(3):219–229. doi: 10.1016/s0174-173x(84)80044-8. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Rosenberg L. An immunoelectron microscope study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage. J Cell Biol. 1982 Jun;93(3):921–937. doi: 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Reiner A., Tang L. H., Rosenberg L. Proteoglycans from bovine nasal cartilage. Immunochemical studies of link protein. J Biol Chem. 1980 Oct 10;255(19):9295–9305. [PubMed] [Google Scholar]
- Smith B. D., Martin G. R., Miller E. J., Dorfman A., Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975 Jan;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- Stark M., Miller E. J., Kühn K. Comparative electron-microscope studies on the collagens extracted from cartilage, bone, and skin. Eur J Biochem. 1972 May;27(1):192–196. doi: 10.1111/j.1432-1033.1972.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Lowther D. A. The effect of chondroitin sulphate-protein on the formation of collagen fibrils in vitro. Biochem J. 1968 Oct;109(5):857–866. doi: 10.1042/bj1090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L. Multistep assembly of type I collagen fibrils. Cell. 1982 Feb;28(2):197–198. doi: 10.1016/0092-8674(82)90334-8. [DOI] [PubMed] [Google Scholar]
- WOOD G. C. The formation of fibrils from collagen solutions. 3. Effect of chondroitin sulphate and some other naturally occurring polyanions on the rate of formation. Biochem J. 1960 Jun;75:605–612. doi: 10.1042/bj0750605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. R., Gelman R. A., Poppke D. C., Piez K. A. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem. 1978 Sep 25;253(18):6578–6585. [PubMed] [Google Scholar]