Abstract
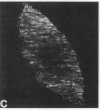
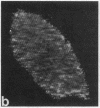
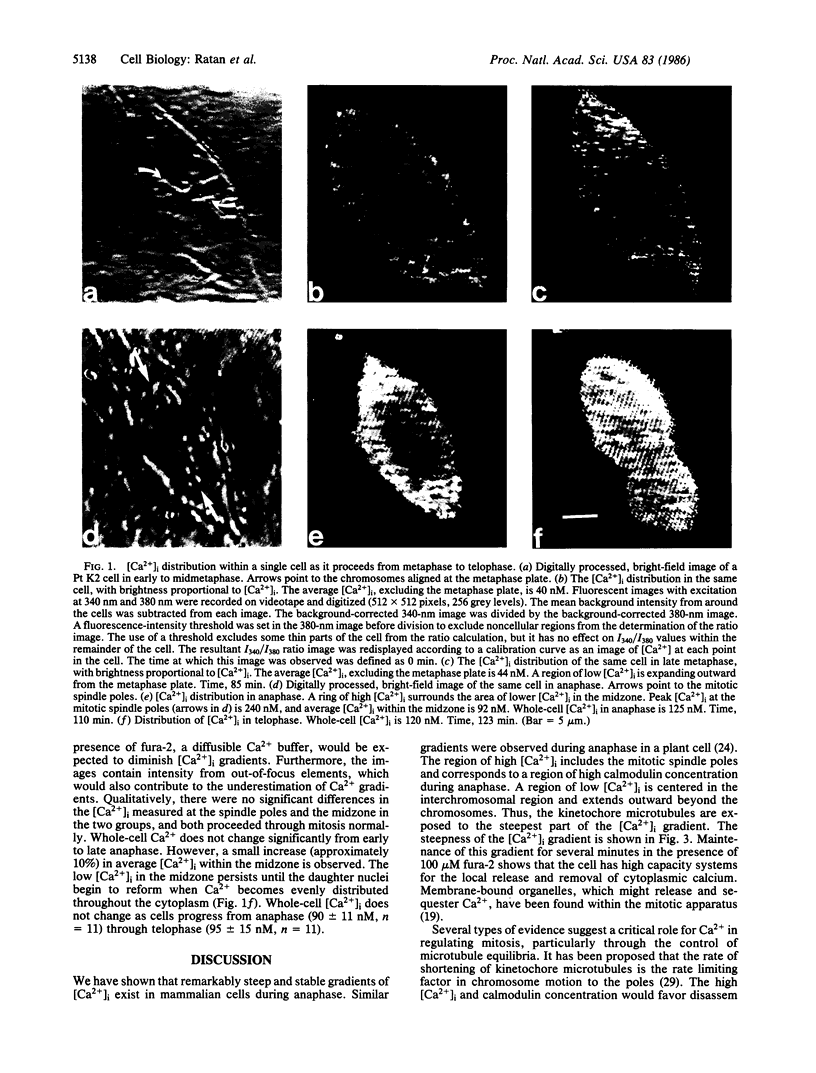
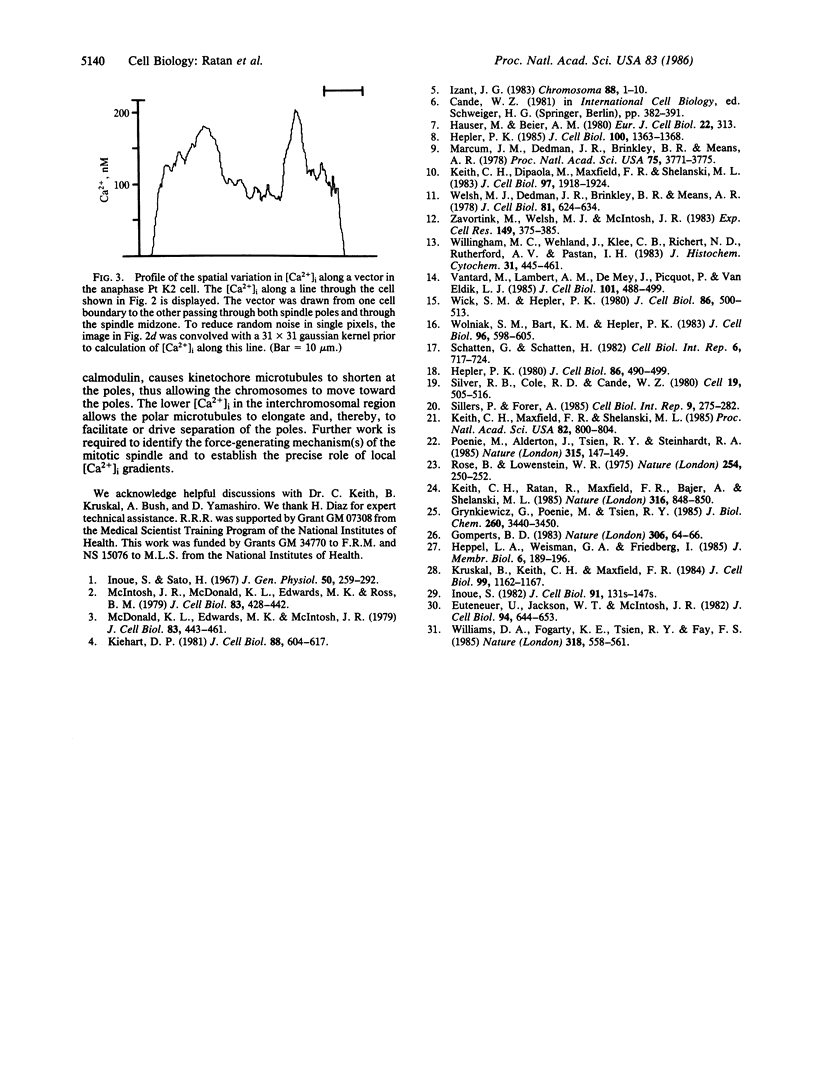
We have used a Ca2+-sensitive dye, fura-2, to investigate the role of Ca2+ during mitosis in Pt K2 epithelial cells. The concentration of cytoplasmic free calcium, [Ca2+]i, increased 2-fold between metaphase and anaphase. Digital image analysis revealed two patterns of [Ca2+]i localization during anaphase. In half of the anaphase cells, the increase in [Ca2+]i was greatest in the region near the spindle poles and decreased radially. In the other anaphase cells, there was a ring of high [Ca2+]i in the cytoplasm, surrounding an area of low [Ca2+]i in the spindle midzone. Although the reason for the different patterns is not known, peak [Ca2+]i in both cases was sufficient to maintain a 2- to 6-fold gradient in [Ca2+]i from the polar region to the midzone. [Ca2+]i gradients may thus regulate spindle microtubule equilibria and directed chromosome movement during mitosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Euteneuer U., Jackson W. T., McIntosh J. R. Polarity of spindle microtubules in Haemanthus endosperm. J Cell Biol. 1982 Sep;94(3):644–653. doi: 10.1083/jcb.94.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hepler P. K. Calcium restriction prolongs metaphase in dividing Tradescantia stamen hair cells. J Cell Biol. 1985 May;100(5):1363–1368. doi: 10.1083/jcb.100.5.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P. K. Membranes in the mitotic apparatus of barley cells. J Cell Biol. 1980 Aug;86(2):490–499. doi: 10.1083/jcb.86.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A., Weisman G. A., Friedberg I. Permeabilization of transformed cells in culture by external ATP. J Membr Biol. 1985;86(3):189–196. doi: 10.1007/BF01870597. [DOI] [PubMed] [Google Scholar]
- Inoué S. Cell division and the mitotic spindle. J Cell Biol. 1981 Dec;91(3 Pt 2):131s–147s. doi: 10.1083/jcb.91.3.131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Izant J. G. The role of calcium ions during mitosis. Calcium participates in the anaphase trigger. Chromosoma. 1983;88(1):1–10. doi: 10.1007/BF00329497. [DOI] [PubMed] [Google Scholar]
- Keith C. H., Maxfield F. R., Shelanski M. L. Intracellular free calcium levels are reduced in mitotic Pt K2 epithelial cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):800–804. doi: 10.1073/pnas.82.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith C. H., Ratan R., Maxfield F. R., Bajer A., Shelanski M. L. Local cytoplasmic calcium gradients in living mitotic cells. 1985 Aug 29-Sep 4Nature. 316(6031):848–850. doi: 10.1038/316848a0. [DOI] [PubMed] [Google Scholar]
- Keith C., DiPaola M., Maxfield F. R., Shelanski M. L. Microinjection of Ca++-calmodulin causes a localized depolymerization of microtubules. J Cell Biol. 1983 Dec;97(6):1918–1924. doi: 10.1083/jcb.97.6.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart D. P. Studies on the in vivo sensitivity of spindle microtubules to calcium ions and evidence for a vesicular calcium-sequestering system. J Cell Biol. 1981 Mar;88(3):604–617. doi: 10.1083/jcb.88.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal B. A., Keith C. H., Maxfield F. R. Thyrotropin-releasing hormone-induced changes in intracellular [Ca2+] measured by microspectrofluorometry on individual quin2-loaded cells. J Cell Biol. 1984 Sep;99(3):1167–1172. doi: 10.1083/jcb.99.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald K. L., Edwards M. K., McIntosh J. R. Cross-sectional structure of the central mitotic spindle of Diatoma vulgare. Evidence for specific interactions between antiparallel microtubules. J Cell Biol. 1979 Nov;83(2 Pt 1):443–461. doi: 10.1083/jcb.83.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J. R., McDonald K. L., Edwards M. K., Ross B. M. Three-dimensional structure of the central mitotic spindle of Diatoma vulgare. J Cell Biol. 1979 Nov;83(2 Pt 1):428–442. doi: 10.1083/jcb.83.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Schatten G., Schatten H., Simerly C. Detection of sequestered calcium during mitosis in mammalian cell cultures and in mitotic apparatus isolated from sea urchin zygotes. Cell Biol Int Rep. 1982 Aug;6(8):717–724. doi: 10.1016/0309-1651(82)90163-1. [DOI] [PubMed] [Google Scholar]
- Sillers P. J., Forer A. CA++ in fertilization and mitosis: the phosphatidylinositol cycle in sea urchin gametes and zygotes is involved in control of fertilization and mitosis. Cell Biol Int Rep. 1985 Mar;9(3):275–282. doi: 10.1016/0309-1651(85)90044-x. [DOI] [PubMed] [Google Scholar]
- Silver R. B., Cole R. D., Cande W. Z. Isolation of mitotic apparatus containing vesicles with calcium sequestration activity. Cell. 1980 Feb;19(2):505–516. doi: 10.1016/0092-8674(80)90525-5. [DOI] [PubMed] [Google Scholar]
- Vantard M., Lambert A. M., De Mey J., Picquot P., Van Eldik L. J. Characterization and immunocytochemical distribution of calmodulin in higher plant endosperm cells: localization in the mitotic apparatus. J Cell Biol. 1985 Aug;101(2):488–499. doi: 10.1083/jcb.101.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Tubulin and calmodulin. Effects of microtubule and microfilament inhibitors on localization in the mitotic apparatus. J Cell Biol. 1979 Jun;81(3):624–634. doi: 10.1083/jcb.81.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick S. M., Hepler P. K. Localization of Ca++-containing antimonate precipitates during mitosis. J Cell Biol. 1980 Aug;86(2):500–513. doi: 10.1083/jcb.86.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Wehland J., Klee C. B., Richert N. D., Rutherford A. V., Pastan I. H. Ultrastructural immunocytochemical localization of calmodulin in cultured cells. J Histochem Cytochem. 1983 Apr;31(4):445–461. doi: 10.1177/31.4.6338107. [DOI] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. Ionic changes in the mitotic apparatus at the metaphase/anaphase transition. J Cell Biol. 1983 Mar;96(3):598–605. doi: 10.1083/jcb.96.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavortink M., Welsh M. J., McIntosh J. R. The distribution of calmodulin in living mitotic cells. Exp Cell Res. 1983 Dec;149(2):375–385. doi: 10.1016/0014-4827(83)90350-6. [DOI] [PubMed] [Google Scholar]