Abstract
In the title compound, C24H20ClF2N3O3S, the essentially planar triazole ring (r.m.s. deviation = 0.001 Å) forms dihedral angles of 22.35 (10), 68.17 (10) and 42.01 (10)° with the mean planes of the trimethoxyphenyl, chlorophenyl and difluorophenyl rings, respectively. A weak intramolecular C—H⋯π interaction occurs. In the crystal, molecules are linked into sheets lying parallel to the bc plane by C—H⋯O and C—H⋯N hydrogen bonds. The crystal packing also features weak C—H⋯π interactions.
Related literature
For the pharmacological activity of [1,2,4] triazole derivatives, see: Zhou et al. (2007 ▶); Chen et al. (2007 ▶); Isloor et al. (2010 ▶); Kalluraya et al. (2004 ▶); Sunil et al. (2009 ▶); Chandrakantha et al. (2010 ▶). For stability of the temperature controller used in the data collection, see: Cosier & Glazer (1986 ▶).
Experimental
Crystal data
C24H20ClF2N3O3S
M r = 503.94
Monoclinic,
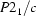
a = 9.9867 (2) Å
b = 21.5140 (3) Å
c = 11.9793 (2) Å
β = 117.197 (1)°
V = 2289.24 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.31 mm−1
T = 100 K
0.36 × 0.17 × 0.11 mm
Data collection
Bruker SMART APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.896, T max = 0.968
26235 measured reflections
6681 independent reflections
5130 reflections with I > 2σ(I)
R int = 0.039
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.110
S = 1.03
6681 reflections
310 parameters
H-atom parameters constrained
Δρmax = 0.43 e Å−3
Δρmin = −0.33 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811048653/hb6503sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811048653/hb6503Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811048653/hb6503Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg2 and Cg3 are the centroids of the C1–C6 and C9–C14 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11A⋯O2i | 0.95 | 2.43 | 3.353 (2) | 165 |
| C15—H15B⋯O3ii | 0.99 | 2.54 | 3.281 (2) | 132 |
| C24—H24A⋯N2iii | 0.98 | 2.49 | 3.189 (2) | 128 |
| C20—H20A⋯Cg2iv | 0.95 | 2.66 | 3.543 (2) | 154 |
| C1—H1A⋯Cg3 | 0.95 | 2.85 | 3.6138 (19) | 138 |
Symmetry codes: (i) 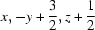 ; (ii)
; (ii) 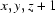 ; (iii)
; (iii)  ; (iv)
; (iv) 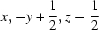 .
.
Acknowledgments
HKF and SIJA thank the Malaysian Government and Universiti Sains Malaysia for the Research University Grants (Nos.1001/PFIZIK/811160 and 1001/PFIZIK/ 811151). AMI thanks the Board for Research in Nuclear Sciences, Department of Atomic Energy, and the Government of India for the Young Scientist award.
supplementary crystallographic information
Comment
During the last few decades, a considerable attention has been devoted to the synthesis of [1, 2, 4] triazole derivatives possessing such diverse pharmacological properties as antimicrobial, anti-inflammatory (Zhou et al., 2007), analgesic antitumorial, antihypertensive (Chen et al., 2007), anticonvulsant and antiviral activities (Isloor et al., 2010). Some 1, 2, 4-triazoles are used as DNA cleaving agents and potassium channel activators. Introduction of fluorine atom in these compounds could alter the course of the pharmacological activities (Kalluraya et al., 2004). In particular, introduction of diflurophenyl substituted group in the moiety immensely increases the pharmacological as well liphophilicity effectiveness (Sunil et al., 2009). It is also observed that the amino and mercapto groups in triazoles are readily accessible nucleophilic centers (Chandrakantha et al., 2010).
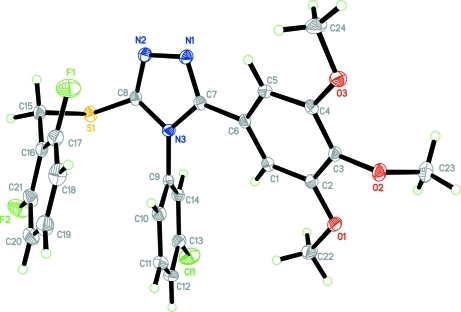
In the title compound of (I), (Fig. 1), the triazole (N1–N3/C7/C8) ring is essentially planar, with maximum deviation of 0.001 Å for C1 and N2. The dihedral angles between triazole ring and the mean plane of trimethoxyphenyl (C1–C6/C22–C24/O1–O3), chlorophenyl (C9–C14/Cl1), and difluorophenyl groups (C16–C21/F1–F2) are 22.35 (10), 68.17 (10) and 42.01 (10)° respectively.
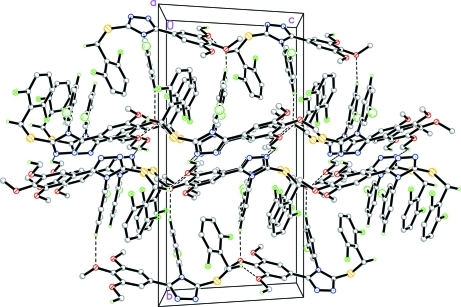
In the crystal structure of (Fig. 2), the molecules are linked into two-dimensional network parallel to bc plane by C11—H11A···O2, C15—H15B···O3 and C24—H24A···N2 hydrogen bonds. The crystal packing is further stabilized by weak C—H···π interactions (Table 1) with distances of 3.543 (2) and 3.6138 (19) A.
Experimental
To a solution of 4-(3-chloro phenyl)-5-(3,4,5-trimethoxy phenyl) -4H-1,2,4-triazole-3-thiol (1 g, 0.0026 mol) in dry acetonitrile (20 ml) was added potassium carbonate (0.73 g, 0.0053 mol) followed by 2,6-difluorobenzyl bromide (0.58 g, 0.0029 mol) at RT. After the addition, the reaction mixture was stirred at RT for 6h. Reaction mixture was monitored by TLC. After the completion, the reaction mixture was concentrated and purified by column chromatography using pet ether, ethyl acetate as an eluent to afford title compound as colorless solid. Yield: 1.1 g, 84%. M.p. 450-453 K.
Refinement
All the H atoms were positioned geometrically and refined using a riding model with C–H = 0.93–0.99 Å. The Uiso values were constrained to be 1.5Ueq of the carrier atom for methyl H atoms and 1.2Ueq for the remaining H atoms. A rotating group model was used for the methyl groups.
Figures
Fig. 1.
The structure of the title compound, showing 50% probability displacement ellipsoids. Hydrogen atoms are shown as spheres of arbitrary radius.
Fig. 2.
The crystal packing, viewed along the a-axis, showing two-dimensional planes parallel to bc plane. Hydrogen atoms that not involved in hydrogen bonding (dashed lines) are omitted for clarity.
Crystal data
| C24H20ClF2N3O3S | F(000) = 1040 |
| Mr = 503.94 | Dx = 1.462 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 7706 reflections |
| a = 9.9867 (2) Å | θ = 2.3–30.0° |
| b = 21.5140 (3) Å | µ = 0.31 mm−1 |
| c = 11.9793 (2) Å | T = 100 K |
| β = 117.197 (1)° | Block, colourless |
| V = 2289.24 (7) Å3 | 0.36 × 0.17 × 0.11 mm |
| Z = 4 |
Data collection
| Bruker SMART APEXII CCD diffractometer | 6681 independent reflections |
| Radiation source: fine-focus sealed tube | 5130 reflections with I > 2σ(I) |
| graphite | Rint = 0.039 |
| φ and ω scans | θmax = 30.0°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −13→14 |
| Tmin = 0.896, Tmax = 0.968 | k = −30→28 |
| 26235 measured reflections | l = −16→13 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.049 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.110 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0429P)2 + 1.4878P] where P = (Fo2 + 2Fc2)/3 |
| 6681 reflections | (Δ/σ)max < 0.001 |
| 310 parameters | Δρmax = 0.43 e Å−3 |
| 0 restraints | Δρmin = −0.33 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.09590 (5) | 0.86248 (3) | 0.08433 (5) | 0.03151 (13) | |
| S1 | 0.68053 (5) | 0.94142 (2) | 0.38418 (4) | 0.01701 (10) | |
| F1 | 1.00621 (13) | 0.87358 (6) | 0.32771 (11) | 0.0301 (3) | |
| F2 | 0.68850 (14) | 0.78856 (5) | 0.47897 (11) | 0.0313 (3) | |
| O1 | 0.29452 (13) | 0.86279 (6) | −0.34883 (11) | 0.0175 (3) | |
| O2 | 0.48997 (14) | 0.88263 (6) | −0.44702 (11) | 0.0175 (3) | |
| O3 | 0.75145 (14) | 0.93893 (6) | −0.31485 (11) | 0.0182 (3) | |
| N1 | 0.77291 (16) | 0.99061 (7) | 0.11532 (13) | 0.0162 (3) | |
| N2 | 0.79349 (16) | 0.99452 (7) | 0.23776 (13) | 0.0163 (3) | |
| N3 | 0.61625 (16) | 0.92492 (6) | 0.13604 (13) | 0.0137 (3) | |
| C1 | 0.47268 (19) | 0.90647 (8) | −0.14903 (15) | 0.0147 (3) | |
| H1A | 0.4059 | 0.8999 | −0.1136 | 0.018* | |
| C2 | 0.42908 (18) | 0.89043 (8) | −0.27358 (15) | 0.0146 (3) | |
| C3 | 0.52434 (19) | 0.90190 (8) | −0.32781 (15) | 0.0139 (3) | |
| C4 | 0.66518 (19) | 0.92956 (8) | −0.25484 (15) | 0.0143 (3) | |
| C5 | 0.71188 (18) | 0.94367 (8) | −0.12895 (15) | 0.0146 (3) | |
| H5A | 0.8088 | 0.9609 | −0.0791 | 0.018* | |
| C6 | 0.61431 (19) | 0.93212 (8) | −0.07673 (15) | 0.0136 (3) | |
| C7 | 0.66734 (18) | 0.94915 (8) | 0.05602 (15) | 0.0137 (3) | |
| C8 | 0.69995 (18) | 0.95528 (8) | 0.24881 (15) | 0.0143 (3) | |
| C9 | 0.50775 (19) | 0.87636 (8) | 0.11320 (14) | 0.0139 (3) | |
| C10 | 0.5402 (2) | 0.81642 (8) | 0.09147 (16) | 0.0180 (3) | |
| H10A | 0.6346 | 0.8069 | 0.0944 | 0.022* | |
| C11 | 0.4324 (2) | 0.77023 (9) | 0.06527 (16) | 0.0216 (4) | |
| H11A | 0.4528 | 0.7289 | 0.0494 | 0.026* | |
| C12 | 0.2952 (2) | 0.78418 (9) | 0.06222 (17) | 0.0223 (4) | |
| H12A | 0.2215 | 0.7527 | 0.0442 | 0.027* | |
| C13 | 0.2666 (2) | 0.84463 (9) | 0.08571 (16) | 0.0196 (4) | |
| C14 | 0.37204 (19) | 0.89165 (8) | 0.11156 (15) | 0.0156 (3) | |
| H14A | 0.3518 | 0.9329 | 0.1276 | 0.019* | |
| C15 | 0.8492 (2) | 0.89379 (8) | 0.47096 (16) | 0.0180 (4) | |
| H15A | 0.9389 | 0.9180 | 0.4825 | 0.022* | |
| H15B | 0.8586 | 0.8847 | 0.5553 | 0.022* | |
| C16 | 0.84819 (19) | 0.83360 (8) | 0.40752 (15) | 0.0174 (3) | |
| C17 | 0.9269 (2) | 0.82486 (9) | 0.33895 (16) | 0.0201 (4) | |
| C18 | 0.9289 (2) | 0.76939 (9) | 0.28106 (17) | 0.0251 (4) | |
| H18A | 0.9852 | 0.7655 | 0.2354 | 0.030* | |
| C19 | 0.8471 (2) | 0.71991 (9) | 0.29131 (18) | 0.0270 (4) | |
| H19A | 0.8467 | 0.6815 | 0.2521 | 0.032* | |
| C20 | 0.7655 (2) | 0.72574 (9) | 0.35825 (17) | 0.0256 (4) | |
| H20A | 0.7092 | 0.6918 | 0.3657 | 0.031* | |
| C21 | 0.7685 (2) | 0.78214 (9) | 0.41376 (16) | 0.0208 (4) | |
| C22 | 0.2031 (2) | 0.84505 (9) | −0.29081 (17) | 0.0204 (4) | |
| H22A | 0.1137 | 0.8230 | −0.3518 | 0.031* | |
| H22B | 0.1720 | 0.8823 | −0.2616 | 0.031* | |
| H22C | 0.2609 | 0.8178 | −0.2193 | 0.031* | |
| C23 | 0.3695 (2) | 0.91553 (10) | −0.54687 (16) | 0.0236 (4) | |
| H23A | 0.3463 | 0.8955 | −0.6272 | 0.035* | |
| H23B | 0.4001 | 0.9587 | −0.5485 | 0.035* | |
| H23C | 0.2801 | 0.9148 | −0.5331 | 0.035* | |
| C24 | 0.8921 (2) | 0.97061 (9) | −0.24538 (18) | 0.0237 (4) | |
| H24A | 0.9396 | 0.9779 | −0.2999 | 0.036* | |
| H24B | 0.9586 | 0.9450 | −0.1737 | 0.036* | |
| H24C | 0.8740 | 1.0105 | −0.2151 | 0.036* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0222 (2) | 0.0397 (3) | 0.0385 (3) | −0.0091 (2) | 0.0190 (2) | −0.0078 (2) |
| S1 | 0.0213 (2) | 0.0192 (2) | 0.01326 (19) | 0.00148 (17) | 0.01018 (16) | −0.00077 (16) |
| F1 | 0.0286 (6) | 0.0313 (7) | 0.0378 (7) | −0.0063 (5) | 0.0217 (5) | −0.0054 (5) |
| F2 | 0.0480 (8) | 0.0257 (6) | 0.0333 (6) | −0.0054 (5) | 0.0299 (6) | −0.0001 (5) |
| O1 | 0.0166 (6) | 0.0213 (7) | 0.0138 (6) | −0.0053 (5) | 0.0063 (5) | −0.0016 (5) |
| O2 | 0.0221 (6) | 0.0185 (6) | 0.0120 (5) | 0.0011 (5) | 0.0080 (5) | −0.0016 (5) |
| O3 | 0.0198 (6) | 0.0215 (6) | 0.0170 (6) | −0.0054 (5) | 0.0115 (5) | −0.0030 (5) |
| N1 | 0.0173 (7) | 0.0187 (8) | 0.0126 (7) | −0.0025 (6) | 0.0068 (6) | −0.0017 (5) |
| N2 | 0.0183 (7) | 0.0172 (7) | 0.0131 (6) | −0.0016 (6) | 0.0070 (6) | −0.0012 (5) |
| N3 | 0.0149 (6) | 0.0134 (7) | 0.0129 (6) | −0.0018 (5) | 0.0064 (5) | −0.0013 (5) |
| C1 | 0.0160 (8) | 0.0144 (8) | 0.0136 (7) | 0.0010 (6) | 0.0066 (6) | 0.0020 (6) |
| C2 | 0.0147 (8) | 0.0132 (8) | 0.0139 (8) | 0.0007 (6) | 0.0048 (6) | 0.0015 (6) |
| C3 | 0.0176 (8) | 0.0125 (8) | 0.0109 (7) | 0.0010 (6) | 0.0060 (6) | −0.0002 (6) |
| C4 | 0.0164 (8) | 0.0145 (8) | 0.0143 (8) | −0.0001 (6) | 0.0091 (6) | 0.0016 (6) |
| C5 | 0.0152 (8) | 0.0130 (8) | 0.0148 (8) | −0.0012 (6) | 0.0061 (6) | −0.0001 (6) |
| C6 | 0.0159 (7) | 0.0120 (8) | 0.0121 (7) | 0.0013 (6) | 0.0057 (6) | 0.0015 (6) |
| C7 | 0.0153 (7) | 0.0146 (8) | 0.0122 (7) | 0.0012 (6) | 0.0070 (6) | 0.0010 (6) |
| C8 | 0.0165 (8) | 0.0146 (8) | 0.0120 (7) | 0.0018 (6) | 0.0068 (6) | −0.0004 (6) |
| C9 | 0.0169 (8) | 0.0148 (8) | 0.0092 (7) | −0.0024 (6) | 0.0053 (6) | 0.0012 (6) |
| C10 | 0.0200 (8) | 0.0157 (8) | 0.0161 (8) | 0.0013 (7) | 0.0066 (7) | 0.0005 (7) |
| C11 | 0.0283 (10) | 0.0138 (8) | 0.0188 (8) | −0.0022 (7) | 0.0074 (7) | 0.0004 (7) |
| C12 | 0.0261 (9) | 0.0193 (9) | 0.0190 (9) | −0.0098 (7) | 0.0082 (7) | 0.0005 (7) |
| C13 | 0.0194 (8) | 0.0248 (10) | 0.0157 (8) | −0.0039 (7) | 0.0091 (7) | 0.0001 (7) |
| C14 | 0.0189 (8) | 0.0155 (8) | 0.0135 (8) | −0.0013 (6) | 0.0084 (6) | −0.0003 (6) |
| C15 | 0.0219 (9) | 0.0170 (9) | 0.0122 (8) | 0.0002 (7) | 0.0052 (7) | −0.0004 (6) |
| C16 | 0.0190 (8) | 0.0162 (8) | 0.0125 (8) | 0.0018 (7) | 0.0034 (6) | 0.0006 (6) |
| C17 | 0.0185 (8) | 0.0215 (9) | 0.0184 (8) | −0.0004 (7) | 0.0068 (7) | 0.0001 (7) |
| C18 | 0.0259 (10) | 0.0288 (11) | 0.0206 (9) | 0.0078 (8) | 0.0105 (8) | −0.0015 (8) |
| C19 | 0.0343 (11) | 0.0197 (10) | 0.0210 (9) | 0.0074 (8) | 0.0075 (8) | −0.0015 (7) |
| C20 | 0.0332 (11) | 0.0176 (9) | 0.0217 (9) | 0.0011 (8) | 0.0087 (8) | 0.0032 (7) |
| C21 | 0.0285 (10) | 0.0198 (9) | 0.0160 (8) | 0.0023 (7) | 0.0118 (7) | 0.0030 (7) |
| C22 | 0.0168 (8) | 0.0252 (10) | 0.0196 (9) | −0.0050 (7) | 0.0087 (7) | −0.0011 (7) |
| C23 | 0.0211 (9) | 0.0335 (11) | 0.0142 (8) | 0.0018 (8) | 0.0065 (7) | 0.0021 (7) |
| C24 | 0.0233 (9) | 0.0285 (10) | 0.0242 (9) | −0.0108 (8) | 0.0151 (8) | −0.0085 (8) |
Geometric parameters (Å, °)
| Cl1—C13 | 1.7404 (19) | C10—H10A | 0.9500 |
| S1—C8 | 1.7446 (17) | C11—C12 | 1.386 (3) |
| S1—C15 | 1.8352 (18) | C11—H11A | 0.9500 |
| F1—C17 | 1.357 (2) | C12—C13 | 1.388 (3) |
| F2—C21 | 1.355 (2) | C12—H12A | 0.9500 |
| O1—C2 | 1.366 (2) | C13—C14 | 1.389 (2) |
| O1—C22 | 1.429 (2) | C14—H14A | 0.9500 |
| O2—C3 | 1.3714 (19) | C15—C16 | 1.499 (2) |
| O2—C23 | 1.436 (2) | C15—H15A | 0.9900 |
| O3—C4 | 1.366 (2) | C15—H15B | 0.9900 |
| O3—C24 | 1.436 (2) | C16—C17 | 1.385 (3) |
| N1—C7 | 1.314 (2) | C16—C21 | 1.386 (3) |
| N1—N2 | 1.3875 (19) | C17—C18 | 1.385 (3) |
| N2—C8 | 1.310 (2) | C18—C19 | 1.381 (3) |
| N3—C7 | 1.378 (2) | C18—H18A | 0.9500 |
| N3—C8 | 1.385 (2) | C19—C20 | 1.385 (3) |
| N3—C9 | 1.439 (2) | C19—H19A | 0.9500 |
| C1—C6 | 1.391 (2) | C20—C21 | 1.377 (3) |
| C1—C2 | 1.393 (2) | C20—H20A | 0.9500 |
| C1—H1A | 0.9500 | C22—H22A | 0.9800 |
| C2—C3 | 1.397 (2) | C22—H22B | 0.9800 |
| C3—C4 | 1.405 (2) | C22—H22C | 0.9800 |
| C4—C5 | 1.393 (2) | C23—H23A | 0.9800 |
| C5—C6 | 1.400 (2) | C23—H23B | 0.9800 |
| C5—H5A | 0.9500 | C23—H23C | 0.9800 |
| C6—C7 | 1.474 (2) | C24—H24A | 0.9800 |
| C9—C10 | 1.383 (2) | C24—H24B | 0.9800 |
| C9—C14 | 1.386 (2) | C24—H24C | 0.9800 |
| C10—C11 | 1.392 (3) | ||
| C8—S1—C15 | 99.14 (8) | C14—C13—Cl1 | 118.74 (14) |
| C2—O1—C22 | 116.74 (13) | C9—C14—C13 | 117.84 (16) |
| C3—O2—C23 | 115.73 (13) | C9—C14—H14A | 121.1 |
| C4—O3—C24 | 116.76 (13) | C13—C14—H14A | 121.1 |
| C7—N1—N2 | 107.89 (13) | C16—C15—S1 | 113.79 (12) |
| C8—N2—N1 | 107.45 (13) | C16—C15—H15A | 108.8 |
| C7—N3—C8 | 104.48 (13) | S1—C15—H15A | 108.8 |
| C7—N3—C9 | 128.96 (14) | C16—C15—H15B | 108.8 |
| C8—N3—C9 | 126.46 (14) | S1—C15—H15B | 108.8 |
| C6—C1—C2 | 119.75 (15) | H15A—C15—H15B | 107.7 |
| C6—C1—H1A | 120.1 | C17—C16—C21 | 114.80 (16) |
| C2—C1—H1A | 120.1 | C17—C16—C15 | 122.94 (16) |
| O1—C2—C1 | 123.12 (15) | C21—C16—C15 | 122.26 (16) |
| O1—C2—C3 | 116.34 (14) | F1—C17—C18 | 118.26 (17) |
| C1—C2—C3 | 120.54 (15) | F1—C17—C16 | 117.97 (16) |
| O2—C3—C2 | 122.08 (15) | C18—C17—C16 | 123.77 (18) |
| O2—C3—C4 | 118.62 (15) | C19—C18—C17 | 118.37 (18) |
| C2—C3—C4 | 119.10 (15) | C19—C18—H18A | 120.8 |
| O3—C4—C5 | 123.91 (15) | C17—C18—H18A | 120.8 |
| O3—C4—C3 | 115.34 (14) | C18—C19—C20 | 120.65 (18) |
| C5—C4—C3 | 120.70 (15) | C18—C19—H19A | 119.7 |
| C4—C5—C6 | 119.21 (15) | C20—C19—H19A | 119.7 |
| C4—C5—H5A | 120.4 | C21—C20—C19 | 118.10 (18) |
| C6—C5—H5A | 120.4 | C21—C20—H20A | 121.0 |
| C1—C6—C5 | 120.63 (15) | C19—C20—H20A | 121.0 |
| C1—C6—C7 | 122.13 (15) | F2—C21—C20 | 118.68 (17) |
| C5—C6—C7 | 117.23 (15) | F2—C21—C16 | 117.01 (16) |
| N1—C7—N3 | 109.96 (14) | C20—C21—C16 | 124.31 (18) |
| N1—C7—C6 | 123.75 (15) | O1—C22—H22A | 109.5 |
| N3—C7—C6 | 126.29 (15) | O1—C22—H22B | 109.5 |
| N2—C8—N3 | 110.22 (14) | H22A—C22—H22B | 109.5 |
| N2—C8—S1 | 126.26 (13) | O1—C22—H22C | 109.5 |
| N3—C8—S1 | 123.52 (13) | H22A—C22—H22C | 109.5 |
| C10—C9—C14 | 122.02 (16) | H22B—C22—H22C | 109.5 |
| C10—C9—N3 | 119.47 (15) | O2—C23—H23A | 109.5 |
| C14—C9—N3 | 118.49 (15) | O2—C23—H23B | 109.5 |
| C9—C10—C11 | 118.98 (17) | H23A—C23—H23B | 109.5 |
| C9—C10—H10A | 120.5 | O2—C23—H23C | 109.5 |
| C11—C10—H10A | 120.5 | H23A—C23—H23C | 109.5 |
| C12—C11—C10 | 120.31 (17) | H23B—C23—H23C | 109.5 |
| C12—C11—H11A | 119.8 | O3—C24—H24A | 109.5 |
| C10—C11—H11A | 119.8 | O3—C24—H24B | 109.5 |
| C11—C12—C13 | 119.34 (17) | H24A—C24—H24B | 109.5 |
| C11—C12—H12A | 120.3 | O3—C24—H24C | 109.5 |
| C13—C12—H12A | 120.3 | H24A—C24—H24C | 109.5 |
| C12—C13—C14 | 121.50 (17) | H24B—C24—H24C | 109.5 |
| C12—C13—Cl1 | 119.76 (14) | ||
| C7—N1—N2—C8 | 0.07 (18) | C9—N3—C8—N2 | 176.61 (15) |
| C22—O1—C2—C1 | −5.8 (2) | C7—N3—C8—S1 | −179.61 (12) |
| C22—O1—C2—C3 | 173.75 (15) | C9—N3—C8—S1 | −3.0 (2) |
| C6—C1—C2—O1 | 177.28 (15) | C15—S1—C8—N2 | −76.25 (16) |
| C6—C1—C2—C3 | −2.3 (2) | C15—S1—C8—N3 | 103.35 (15) |
| C23—O2—C3—C2 | 71.3 (2) | C7—N3—C9—C10 | 65.5 (2) |
| C23—O2—C3—C4 | −113.93 (18) | C8—N3—C9—C10 | −110.22 (19) |
| O1—C2—C3—O2 | −4.5 (2) | C7—N3—C9—C14 | −113.38 (19) |
| C1—C2—C3—O2 | 175.11 (15) | C8—N3—C9—C14 | 70.9 (2) |
| O1—C2—C3—C4 | −179.25 (15) | C14—C9—C10—C11 | 1.0 (2) |
| C1—C2—C3—C4 | 0.3 (2) | N3—C9—C10—C11 | −177.84 (15) |
| C24—O3—C4—C5 | −6.1 (2) | C9—C10—C11—C12 | −0.6 (3) |
| C24—O3—C4—C3 | 176.26 (15) | C10—C11—C12—C13 | −0.1 (3) |
| O2—C3—C4—O3 | 4.8 (2) | C11—C12—C13—C14 | 0.4 (3) |
| C2—C3—C4—O3 | 179.76 (15) | C11—C12—C13—Cl1 | −179.09 (14) |
| O2—C3—C4—C5 | −172.94 (15) | C10—C9—C14—C13 | −0.7 (2) |
| C2—C3—C4—C5 | 2.0 (2) | N3—C9—C14—C13 | 178.12 (14) |
| O3—C4—C5—C6 | −179.94 (15) | C12—C13—C14—C9 | 0.0 (3) |
| C3—C4—C5—C6 | −2.4 (2) | Cl1—C13—C14—C9 | 179.49 (12) |
| C2—C1—C6—C5 | 1.9 (2) | C8—S1—C15—C16 | −63.03 (14) |
| C2—C1—C6—C7 | −179.10 (15) | S1—C15—C16—C17 | 100.76 (18) |
| C4—C5—C6—C1 | 0.4 (2) | S1—C15—C16—C21 | −79.58 (19) |
| C4—C5—C6—C7 | −178.63 (15) | C21—C16—C17—F1 | 179.26 (15) |
| N2—N1—C7—N3 | −0.04 (19) | C15—C16—C17—F1 | −1.1 (3) |
| N2—N1—C7—C6 | −179.95 (15) | C21—C16—C17—C18 | −0.5 (3) |
| C8—N3—C7—N1 | 0.00 (18) | C15—C16—C17—C18 | 179.14 (17) |
| C9—N3—C7—N1 | −176.45 (16) | F1—C17—C18—C19 | −179.36 (16) |
| C8—N3—C7—C6 | 179.90 (16) | C16—C17—C18—C19 | 0.4 (3) |
| C9—N3—C7—C6 | 3.5 (3) | C17—C18—C19—C20 | −0.2 (3) |
| C1—C6—C7—N1 | −156.64 (17) | C18—C19—C20—C21 | 0.1 (3) |
| C5—C6—C7—N1 | 22.4 (2) | C19—C20—C21—F2 | 179.65 (17) |
| C1—C6—C7—N3 | 23.5 (3) | C19—C20—C21—C16 | −0.2 (3) |
| C5—C6—C7—N3 | −157.48 (16) | C17—C16—C21—F2 | −179.45 (15) |
| N1—N2—C8—N3 | −0.08 (19) | C15—C16—C21—F2 | 0.9 (3) |
| N1—N2—C8—S1 | 179.57 (12) | C17—C16—C21—C20 | 0.4 (3) |
| C7—N3—C8—N2 | 0.05 (18) | C15—C16—C21—C20 | −179.24 (17) |
Hydrogen-bond geometry (Å, °)
| Cg2 and Cg3 are the centroids of the C1–C6 and C9–C14 rings, respectively. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11A···O2i | 0.95 | 2.43 | 3.353 (2) | 165 |
| C15—H15B···O3ii | 0.99 | 2.54 | 3.281 (2) | 132 |
| C24—H24A···N2iii | 0.98 | 2.49 | 3.189 (2) | 128 |
| C20—H20A···Cg2iv | 0.95 | 2.66 | 3.543 (2) | 154 |
| C1—H1A···Cg3 | 0.95 | 2.85 | 3.6138 (19) | 138 |
Symmetry codes: (i) x, −y+3/2, z+1/2; (ii) x, y, z+1; (iii) −x+2, −y+2, −z; (iv) x, −y+1/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB6503).
References
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Chandrakantha, B., Shetty, P., Nambiyar, V., Isloor, N. & Isloor, A. M. (2010). Eur. J. Med. Chem. 45, 1206–1210. [DOI] [PubMed]
- Chen, C. J., Song, B. A., Yang, S., Xu, G. F., Bhadury, P. S., Jin, L. H., Hu, D. Y., Li, Q. Z., Liu, F., Xue, W., Lu, P. & Chen, Z. (2007). Bioorg. Med. Chem. 15, 3981–3989. [DOI] [PubMed]
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, 105–107.
- Isloor, A. M., Kalluraya, B. & Pai, K. S. (2010). Eur. J. Med. Chem. 45, 825–830. [DOI] [PubMed]
- Kalluraya, B., Jagadeesha, R. L. & Isloor, A. M. (2004). Indian J. Heterocycl. Chem. 13, 245–248.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Sunil, D., Isloor, A. M. & Shetty, P. (2009). Der Pharma Chem. 1, 19–26.
- Zhou, S. N., Zhang, L. X., Zhang, A. J., Sheng, J. S. & Zhang, H. L. (2007). J. Heterocycl. Chem. 44, 1019–1022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811048653/hb6503sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811048653/hb6503Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811048653/hb6503Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report