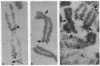
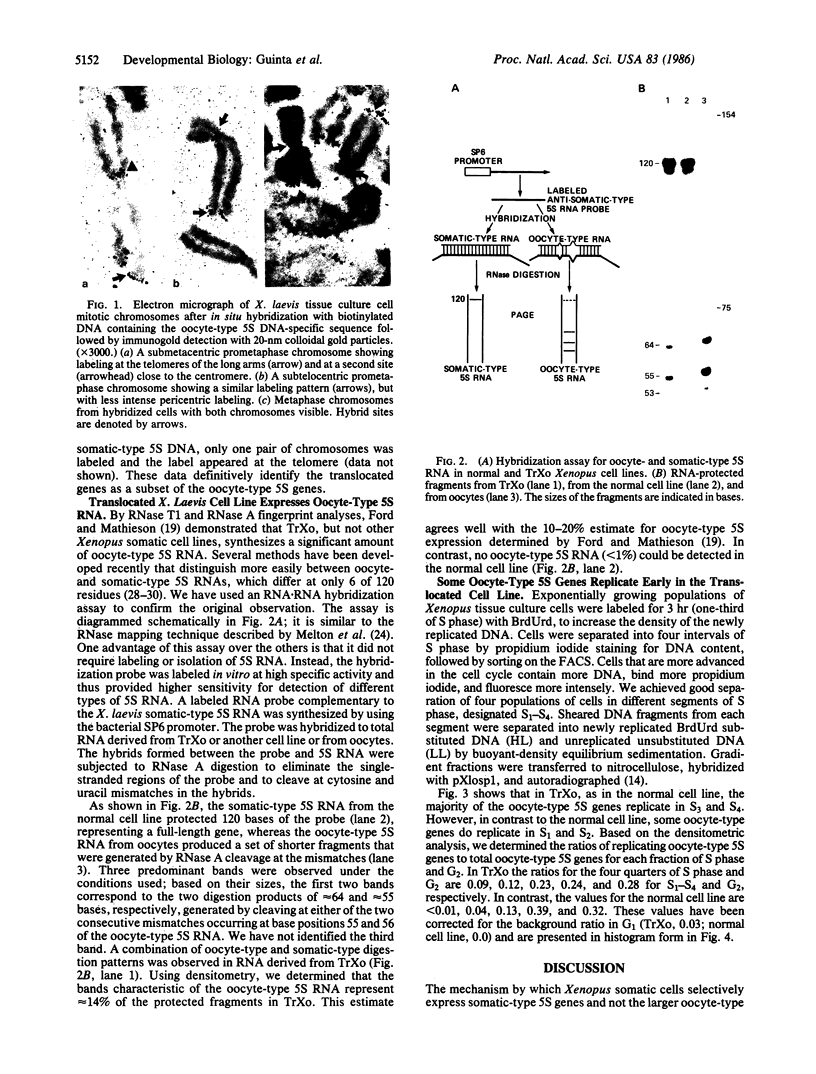
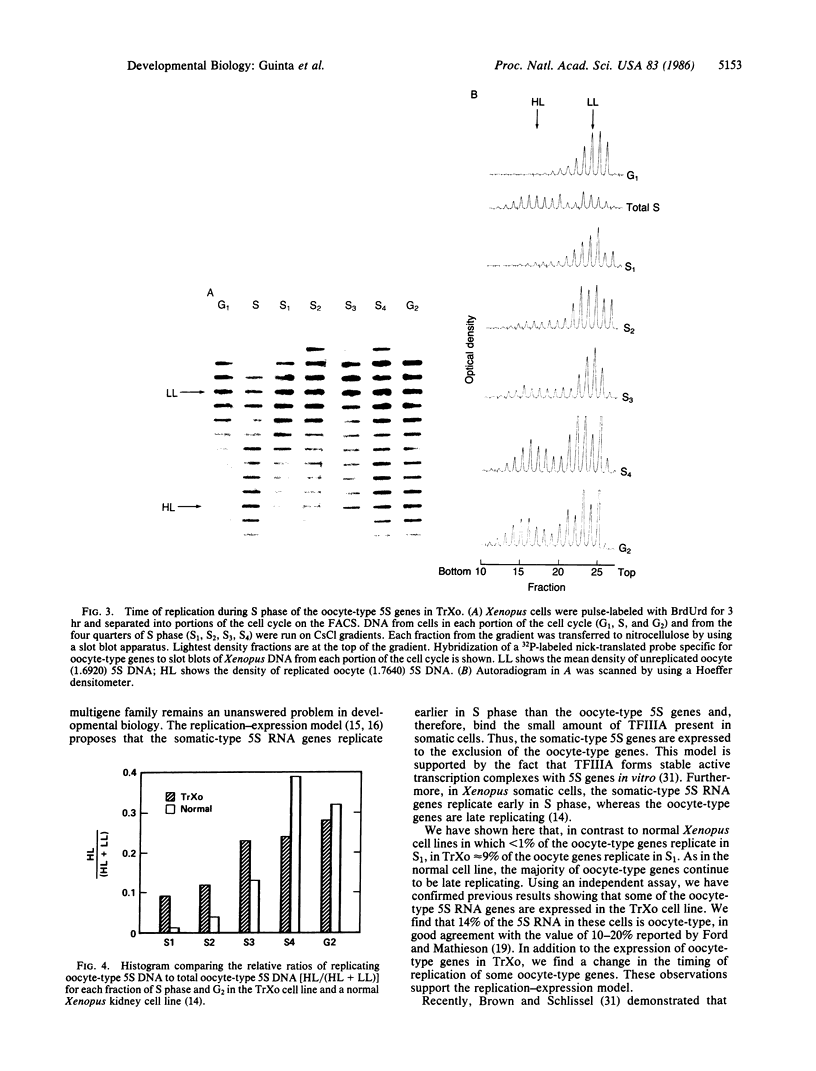
Abstract
In Xenopus somatic cells, the somatic-type 5S RNA genes replicate early in S phase, bind the transcription factor TFIIIA, and are expressed; in contrast, the late replicating oocyte-type genes do not bind TFIIIA and are transcriptionally inactive. These facts support a model in which the order of replication of the somatic-type versus the oocyte-type 5S genes causes their differential expression in somatic cells due to sequestration of TFIIIA by the early-replicating somatic genes. Here we provide further evidence for the model by showing that in one Xenopus cell line in which some oocyte-type 5S genes are translocated, some oocyte-type 5S genes replicate early and are expressed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLOWITZ L. CORRELATION OF GENETIC ACTIVITY, HETEROCHROMATIZATION, AND RNA METABOLISM. Proc Natl Acad Sci U S A. 1965 Jan;53:68–73. doi: 10.1073/pnas.53.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs I., Brown E. H., Schildkraut C. L. The temporal order of replication of some DNA cistrons. Cold Spring Harb Symp Quant Biol. 1974;38:239–245. doi: 10.1101/sqb.1974.038.01.027. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Schlissel M. S. A positive transcription factor controls the differential expression of two 5S RNA genes. Cell. 1985 Oct;42(3):759–767. doi: 10.1016/0092-8674(85)90272-7. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Sugimoto K. The structure and evolution of ribosomal and 5S DNAs in Xenopus laevis and Xenopus mulleri. Cold Spring Harb Symp Quant Biol. 1974;38:501–505. doi: 10.1101/sqb.1974.038.01.054. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Tlsty T. D., Schimke R. T. Enhancement of methotrexate resistance and dihydrofolate reductase gene amplification by treatment of mouse 3T6 cells with hydroxyurea. Mol Cell Biol. 1983 Jun;3(6):1097–1107. doi: 10.1128/mcb.3.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- De Waele M., De Mey J., Moeremans M., De Brabander M., Van Camp B. Immunogold staining method for the light microscopic detection of leukocyte cell surface antigens with monoclonal antibodies: its application to the enumeration of lymphocyte subpopulations. J Histochem Cytochem. 1983 Mar;31(3):376–381. doi: 10.1177/31.3.6186731. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Ng S. Y., Shastry B. S., Roeder R. G. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell. 1980 Mar;19(3):717–728. doi: 10.1016/s0092-8674(80)80048-1. [DOI] [PubMed] [Google Scholar]
- Epner E., Rifkind R. A., Marks P. A. Replication of alpha and beta globin DNA sequences occurs during early S phase in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3058–3062. doi: 10.1073/pnas.78.5.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford P. J., Mathieson T. Control of 5S RNA synthesis in Xenopus laevis. Nature. 1976 Jun 3;261(5559):433–435. doi: 10.1038/261433a0. [DOI] [PubMed] [Google Scholar]
- Ford P. J., Southern E. M. Different sequences for 5S RNA in kidney cells and ovaries of Xenopus laevis. Nat New Biol. 1973 Jan 3;241(105):7–12. doi: 10.1038/newbio241007a0. [DOI] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J., Bloomer L. S. Assembly of transcriptionally active 5S RNA gene chromatin in vitro. Cell. 1982 Apr;28(4):781–791. doi: 10.1016/0092-8674(82)90057-5. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Guinta D. R., Korn L. J. Differential order of replication of Xenopus laevis 5S RNA genes. Mol Cell Biol. 1986 Jul;6(7):2536–2542. doi: 10.1128/mcb.6.7.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamkalo B. A., Farnham P. J., Johnston R., Schimke R. T. Ultrastructural features of minute chromosomes in a methotrexate-resistant mouse 3T3 cell line. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1126–1130. doi: 10.1073/pnas.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Price J., Korn L. J. Chromosomal mapping of Xenopus 5S genes: somatic-type versus oocyte-type. Nucleic Acids Res. 1983 Apr 25;11(8):2313–2323. doi: 10.1093/nar/11.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Huberman J. A. Changes in the rate of DNA replication fork movement during S phase in mammalian cells. J Mol Biol. 1975 May 15;94(2):173–181. doi: 10.1016/0022-2836(75)90076-5. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Gurdon J. B. The reactivation of developmentally inert 5S genes in somatic nuclei injected into Xenopus oocytes. Nature. 1981 Feb 5;289(5797):461–465. doi: 10.1038/289461a0. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Schimke R. T. Gene amplification in a single cell cycle in Chinese hamster ovary cells. J Biol Chem. 1984 Feb 10;259(3):1901–1910. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Brown D. D., Birnstiel M. L. Location of the genes for 5S ribosomal RNA in Xenopus laevis. Chromosoma. 1973;42(2):191–203. doi: 10.1007/BF00320940. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Wormington W. M., Brown D. D. Related 5S RNA transcription factors in Xenopus oocytes and somatic cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1760–1764. doi: 10.1073/pnas.78.3.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. C., Doering J. L., Brown D. D. Characterization of two xenopus somatic 5S DNAs and one minor oocyte-specific 5S DNA. Cell. 1980 May;20(1):131–141. doi: 10.1016/0092-8674(80)90241-x. [DOI] [PubMed] [Google Scholar]
- Rivin C. J., Fangman W. L. Replication fork rate and origin activation during the S phase of Saccharomyces cerevisiae. J Cell Biol. 1980 Apr;85(1):108–115. doi: 10.1083/jcb.85.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M. S., Brown D. D. The transcriptional regulation of Xenopus 5s RNA genes in chromatin: the roles of active stable transcription complexes and histone H1. Cell. 1984 Jul;37(3):903–913. doi: 10.1016/0092-8674(84)90425-2. [DOI] [PubMed] [Google Scholar]
- Wakefield L., Gurdon J. B. Cytoplasmic regulation of 5S RNA genes in nuclear-transplant embryos. EMBO J. 1983;2(9):1613–1619. doi: 10.1002/j.1460-2075.1983.tb01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegnez M., Monier R., Denis H. Sequence heterogeneity of 5 S RNA in Xenopus laevis. FEBS Lett. 1972 Sep 1;25(1):13–20. doi: 10.1016/0014-5793(72)80443-5. [DOI] [PubMed] [Google Scholar]
- Wormington W. M., Schlissel M., Brown D. D. Developmental regulation of Xenopus 5S RNA genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):879–884. doi: 10.1101/sqb.1983.047.01.101. [DOI] [PubMed] [Google Scholar]