Abstract
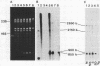
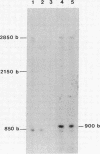
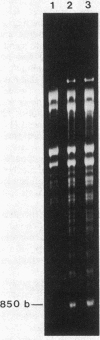
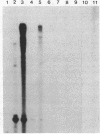
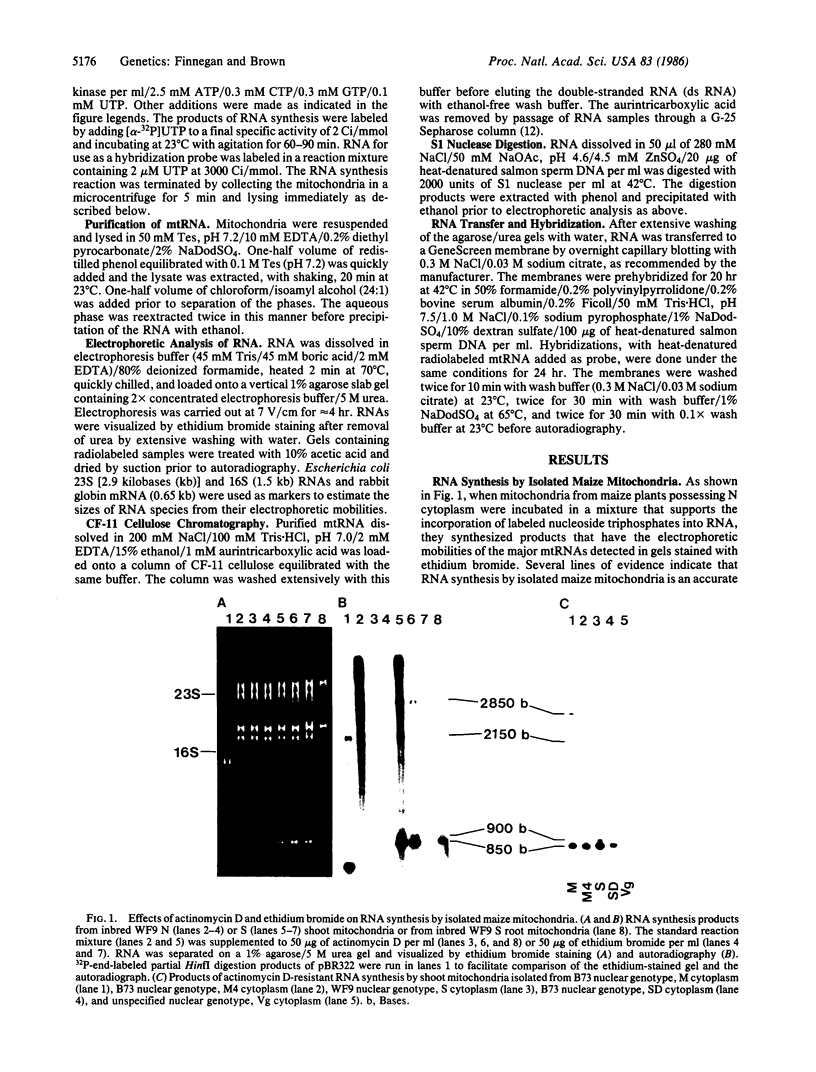
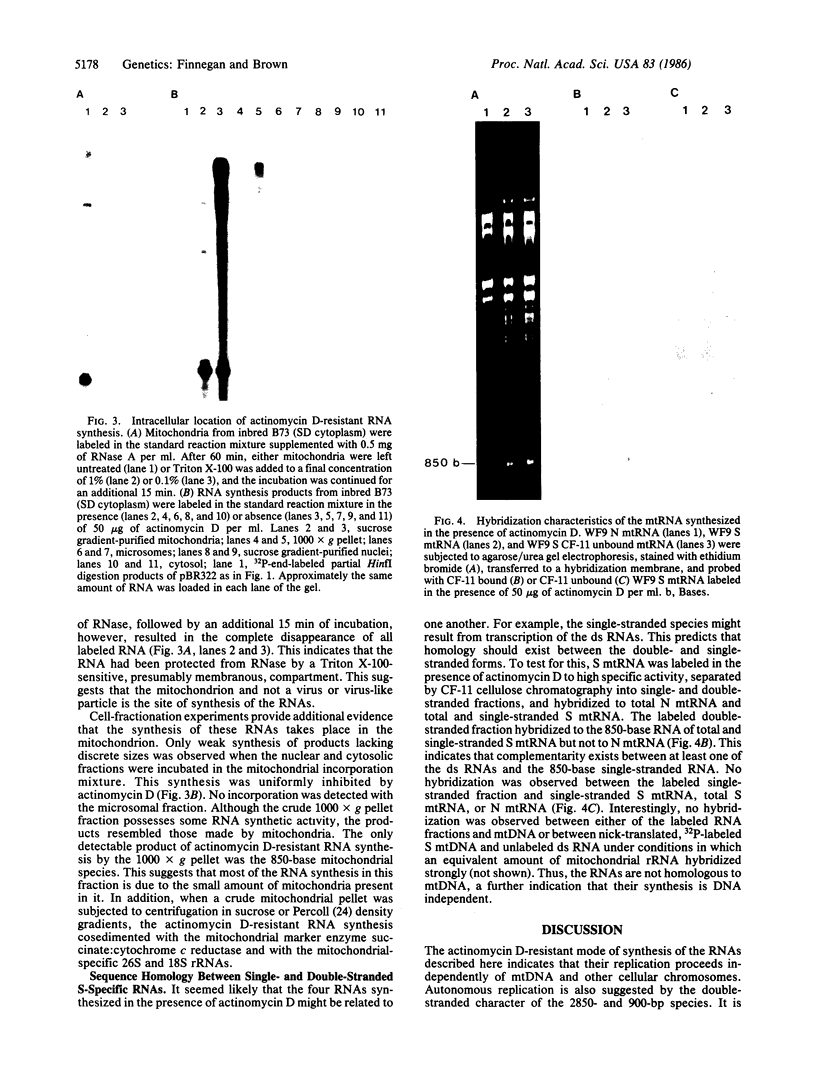
Mitochondria isolated from maize plants with S-type male-sterile cytoplasms are capable of synthesizing four species of RNA at concentrations of actinomycin D that eliminate all DNA-directed RNA synthesis. No RNA synthesis occurs under the same conditions with mitochondria from plants possessing normal (N) cytoplasm or with other subcellular fractions from plants with S cytoplasm. The actinomycin D-resistant RNA synthesis occurs within the mitochondria since the labeling of these species is unaffected by inclusion of RNase in the incubation medium and since they become completely sensitive to RNase upon lysis of the mitochondria with low concentrations of Triton X-100. Two of the actinomycin D-resistant products are double stranded. These are 2850 and 900 base pairs in length, whereas the remaining two are 2150 and 850 bases. The synthesis of all four RNAs occurs in at least five different accessions of S cytoplasm, suggesting it is a general feature of S mitochondria. The double-stranded RNAs show homology to single-stranded S mitochondrial RNA but not to N mitochondrial RNA. Our observations indicate that the replication of these RNAs occurs independently of mtDNA and that they thus represent a novel type of inheritable element in organelles, an RNA plasmid.
Keywords: cytoplasmic male sterility, genetic RNA, RNA plasmids, double-stranded RNA, in organello RNA synthesis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beilharz M. W., Cobon G. S., Nagley P. A novel species of double stranded RNA in mitochondria of Saccharomyces cerevisiae. Nucleic Acids Res. 1982 Feb 11;10(3):1051–1070. doi: 10.1093/nar/10.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner P., Mason T. L., Fox T. D. Synthesis and processing of ribosomal RNA in isolated yeast mitochondria. Nucleic Acids Res. 1981 Dec 11;9(23):6379–6390. doi: 10.1093/nar/9.23.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Wheeler A. M., Whitfeld P. R. The effect of a range of RNA polymerase inhibitors on RNA synthesis in higher plant chloroplasts and nuclei. Arch Biochem Biophys. 1971 Mar;143(1):269–275. doi: 10.1016/0003-9861(71)90209-8. [DOI] [PubMed] [Google Scholar]
- Forde B. G., Leaver C. J. Nuclear and cytoplasmic genes controlling synthesis of variant mitochondrial polypeptides in male-sterile maize. Proc Natl Acad Sci U S A. 1980 Jan;77(1):418–422. doi: 10.1073/pnas.77.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines G., Attardi G. Intercalating drugs and low temperatures inhibit synthesis and processing of ribosomal RNA in isolated human mitochondria. J Mol Biol. 1984 Feb 5;172(4):451–466. doi: 10.1016/s0022-2836(84)80017-0. [DOI] [PubMed] [Google Scholar]
- Groot G. S., van Harten-Loosbroek N., van Ommen G. J., Pijst H. L. RNA synthesis in isolated yeast mitochondria. Nucleic Acids Res. 1981 Dec 11;9(23):6369–6377. doi: 10.1093/nar/9.23.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughnan J. R., Gabay-Laughnan S. Cytoplasmic male sterility in maize. Annu Rev Genet. 1983;17:27–48. doi: 10.1146/annurev.ge.17.120183.000331. [DOI] [PubMed] [Google Scholar]
- Lemke P. A. Viruses of eucaryotic microorganisms. Annu Rev Microbiol. 1976;30:105–145. doi: 10.1146/annurev.mi.30.100176.000541. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Fauron C. M. The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 1984 Dec 21;12(24):9249–9261. doi: 10.1093/nar/12.24.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassuth A., Alblas F., van der Geest A. J., Bol J. F. Inhibition of alfalfa mosaic virus RNA and protein synthesis by actinomycin D and cycloheximide. Virology. 1983 Apr 30;126(2):517–524. doi: 10.1016/s0042-6822(83)80009-9. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Newman D., Martin N. Synthesis of RNA in isolated mitochondria from Saccharomyces cerevisiae. Plasmid. 1982 Jan;7(1):66–76. doi: 10.1016/0147-619x(82)90028-2. [DOI] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S., Hu W. W., Timothy D. H. Unique DNA associated with mitochondria in the "S"-type cytoplasm of male-sterile maize. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2904–2908. doi: 10.1073/pnas.74.7.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederoff R. R. Structural variation in mitochondrial DNA. Adv Genet. 1984;22:1–108. doi: 10.1016/s0065-2660(08)60038-3. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Bostian K. A. Double-stranded ribonucleic acid killer systems in yeasts. Microbiol Rev. 1984 Jun;48(2):125–156. doi: 10.1128/mr.48.2.125-156.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling G. W., Groen G., Welling-Wester S. Isolation of Sendai virus F protein by anion-exchange high-performance liquid chromatography in the presence of Triton X-100. J Chromatogr. 1983 Aug 26;266:629–632. doi: 10.1016/s0021-9673(01)90932-x. [DOI] [PubMed] [Google Scholar]
- Zimmern D. Homologous proteins encoded by yeast mitochondrial introns and by a group of RNA viruses from plants. J Mol Biol. 1983 Dec 15;171(3):345–352. doi: 10.1016/0022-2836(83)90098-0. [DOI] [PubMed] [Google Scholar]