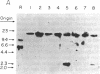
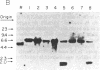
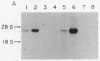
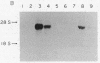
Abstract
Livers from newborn mice homozygous for either one of the lethal deletions c14CoS or c3H in chromosome 7 have drastically reduced levels of cytosolic phosphoenolpyruvate carboxykinase (GTP) [GTP:oxaloacetate carboxy-lyase (transphosphorylating), EC 4.1.1.32] activity when compared with normal littermates. The structural gene for the enzyme maps on chromosome 2 and appears intact and not grossly rearranged in deletion homozygotes. These mice also have negligible levels of hepatic mRNA encoding this enzyme. Studies of the transcription rate of the gene showed that it was reduced to 25-50% of normal in hepatic nuclei obtained from mice homozygous for either deletion. We suggest that, in addition to the reduction in the level of transcription, the deletions in chromosome 7 may also cause alterations in messenger stability, processing, or transport from the nucleus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Hanson R. W. Phosphoenolpyruvate carboxykinase and pyruvate carboxylase in developing rat liver. Biochem J. 1967 Sep;104(3):866–871. doi: 10.1042/bj1040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cori C. F., Gluecksohn-Waelsch S., Klinger H. P., Pick L., Schlagman S. L., Teicher L. S., Wang-Chang H. F. Complementation of gene deletions by cell hybridization. Proc Natl Acad Sci U S A. 1981 Jan;78(1):479–483. doi: 10.1073/pnas.78.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cori C. F., Gluecksohn-Waelsch S., Shaw P. A., Robinson C. Correction of a genetically caused enzyme defect by somatic cell hybridization. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6611–6614. doi: 10.1073/pnas.80.21.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979 Feb;16(2):225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Granner D., Andreone T., Sasaki K., Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983 Oct 6;305(5934):549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- Hanson R. W., Garber A. J. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am J Clin Nutr. 1972 Oct;25(10):1010–1021. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Master regulatory loci in yeast and lambda. Cold Spring Harb Symp Quant Biol. 1985;50:565–574. doi: 10.1101/sqb.1985.050.01.069. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Kioussis D., Hamilton R., Hanson R. W., Tilghman S. M., Taylor J. M. Construction and cloning of rat albumin structural gene sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4370–4374. doi: 10.1073/pnas.76.9.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J., Fournier R. E. Assignment of the gene encoding cytosolic phosphoenolpyruvate carboxykinase (GTP) to Mus musculus chromosome 2. Somat Cell Mol Genet. 1985 Nov;11(6):633–638. doi: 10.1007/BF01534728. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Cameron D. K., Short H. P., Hanson R. W. Thyroid hormone regulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat liver. Biochemistry. 1985 Aug 13;24(17):4509–4512. doi: 10.1021/bi00338a004. [DOI] [PubMed] [Google Scholar]
- Meisner H., Loose D. S., Hanson R. W. Effect of hormones on transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat kidney. Biochemistry. 1985 Jan 15;24(2):421–425. doi: 10.1021/bi00323a027. [DOI] [PubMed] [Google Scholar]
- Meisner H., Polsinelli L. Changes of renal mRNA species abundance by ochratoxin A. Biochem Pharmacol. 1986 Feb 15;35(4):661–665. doi: 10.1016/0006-2952(86)90364-3. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. J., Friedman J. M., Oulette A. J., Krauter K. S., Darnell J. E., Jr Transcriptional and post-transcriptional control of specific messenger RNAs in adult and embryonic liver. J Mol Biol. 1984 Oct 15;179(1):21–35. doi: 10.1016/0022-2836(84)90304-8. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Poiret M., Sellem C. H., Bessada R., Erdos T., Gluecksohn-Waelsch S. A lethal deletion on mouse chromosome 7 affects regulation of liver-cell-specific functions: posttranscriptional control of serum protein and transcriptional control of aldolase B synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2442–2446. doi: 10.1073/pnas.82.8.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid W., Müller G., Schütz G., Gluecksohn-Waelsch S. Deletions near the albino locus on chromosome 7 of the mouse affect the level of tyrosine aminotransferase mRNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2866–2869. doi: 10.1073/pnas.82.9.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike J., Trigg M. J., Stockert R., Gluecksohn-Waelsch S., Cori C. F. Multiple biochemical effects of a series of x-ray induced mutations at the albino locus in the mouse. Biochem Genet. 1973 May;9(1):25–39. doi: 10.1007/BF00485588. [DOI] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Factors affecting the premature induction of phosphopyruvate carboxylase in neonatal rat liver. Biochem J. 1968 Jun;108(2):325–331. doi: 10.1042/bj1080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo-Warren H., Cimbala M. A., Felz K., Monahan J. E., Leis J. P., Hanson R. W. Identification of a DNA clone to phosphoenolpyruvate carboxykinase (GTP) from rat cytosol. Alterations in phosphoenolpyruvate carboxykinase RNA levels detectable by hybridization. J Biol Chem. 1981 Oct 25;256(20):10224–10227. [PubMed] [Google Scholar]
- Yoo-Warren H., Monahan J. E., Short J., Short H., Bruzel A., Wynshaw-Boris A., Meisner H. M., Samols D., Hanson R. W. Isolation and characterization of the gene coding for cytosolic phosphoenolpyruvate carboxykinase (GTP) from the rat. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3656–3660. doi: 10.1073/pnas.80.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]