Abstract
Glutamic acid decarboxylase (GAD) converts glutamic acid into the inhibitory neurotransmitter γ-aminobutyric acid (GABA). Increased serum GAD (auto) antibody concentrations were found in a mare with increased postural musculature tone resulting in stiffness and recumbence. The mare was treated with dexamethasone which resulted in resolution of clinical signs and decreased GAD antibody concentrations.
Résumé
Cas présumé de «syndrome du cheval raide» causé par une production réduite d’acide gamma-aminobutyrique (GABA) chez une jument Paint Horse. La glutamate décarboxylase (GAD) convertit l’acide glutamique en acide gamma-aminobutyrique (GABA), un inhibiteur des neurotransmetteurs. Des concentrations sériques accrues d’anticorps (auto) GAD ont été trouvées chez une jument avec un tonus accru de la musculature de posture se traduisant par de la raideur et un décubitus. La jument a été traitée à la dexaméthasone, ce qui a entrainé une résolution des signes cliniques et une réduction des concentrations d’anticorps GAD.
(Traduit par Isabelle Vallières)
Interneurons in the spinal cord are strategically placed between 2 peripheral nerve reflex loops with antagonizing functions. They have an inhibitory function causing hyperpolarization in the post-synaptic membrane. Neurotransmitters are commonly glycine or γ-aminobutyric acid (GABA). One of the best understood diseases in horses affecting interneurons is tetanus, where the neurotoxin of Clostridium tetani inhibits the release of inhibitory neurotransmitters in the interneuron, thereby allowing antagonizing muscle groups to contract simultaneously (1,2).
Stiff–person syndrome (SPS) is an immune-mediated disease in humans, with antibodies produced against the enzyme glutamic acid decarboxylase (GAD), which is the key enzyme that converts glutamic acid into GABA. In this disease, increased GAD antibody concentrations apparently inactivate the enzyme, which results in muscle stiffness and prolonged muscle contractions of the limb anti-gravity muscles, as well as truncal axial and epaxial musculature. Cerebellar dysfunction has also been described with SPS (3). A similar syndrome in a horse with a diagnosis based on clinical signs, increased GAD antibody titer, and response to corticosteroid therapy has once been described in the veterinary literature in an 11-year-old horse in Europe; the condition was named “stiff–horse syndrome” (SHS) (4).
Case description
This report describes the historical and clinical findings, laboratory results, and treatment of what may be the first documented SHS-case in North America. The owner of a 16-year-old American Paint mare contacted the authors at the Colorado State University (CSU) Veterinary Teaching Hospital in April, 2008, and reported a 4-week history of prolonged recumbence of her horse. The horse had been in the owner’s possession for approximately 6 y, and was used for recreational riding. The mare was the only horse on a 3-acre (12 000 m2) property. Housing consisted of a 14 m2 fenced area with a semi-closed shed (6 m2) that opened to the south. Due to the presenting complaint, recumbence, the owner added a deep layer of sand as additional bedding material to the roofed area. A gate opened into either a fenced, 1.5 acre (6000 m2) pasture, or into a same-size grass-covered area with occasional shrubs or low trees that surrounded the owner’s home.
When recumbence was initially noticed there was no significant growth of vegetation anywhere on the property. The horse was fed grass hay and, occasionally, oats in molasses (sweet feed) purchased from a local dealer, who also sold hay and horse feed to a number of owners of small and mid-sized local horse properties. Hay was available to the horse ad libitum, as was well water, which also supplied the owner’s home. The mare was dewormed twice yearly (ivermectin generic product), and was due for annual vaccinations. Historically, a killed virus vaccine was administered containing antigens from Eastern equine encephalitis, Western equine encephalitis (EEE/WEE), West Nile virus, and tetanus toxoid (West Nile Innovator + EWT, Fort Dodge, Iowa, USA). A history obtained over the phone at that time revealed that the horse was ambulating once or twice during a day for about 10 min. The remainder of the day the mare was recumbent. Laminitis was previously suspected and the horse was treated with phenylbutazone (Bute boluses; VEDCO, St. Joseph, Missouri, USA), 2 g, PO once daily during the 4 wk of prolonged recumbency. On a few occasions a rectal temperature was taken, which, according to the owner, had always been normal. Prior to this 4-week period, the owner reported a tendency in the horse to trip with any forelimb or hind limb when ridden and the mare was more often found resting in sternal recumbency.
Upon presentation the mare was laying on her sternum in the sanded, roofed area. The horse was quiet, alert, and responsive; the body condition was good, and no skin lesions or pressure sores were detected. The body weight was estimated at about 500 kg. Physical examination was performed in the recumbent position, and the parameters assessed were normal. Cranial nerve function was uncompromised; the horse had good cutaneous trunci reflexes, and a strong tail tone with a normal perineal reflex was present. The muscle tone in its limbs appeared strong and a patellar reflex in the upper hind limb could be elicited; however, due to the recumbent position, complete evaluation was difficult. A neurological evaluation was completed in the standing horse. With some stimulation the mare rose with a normal, coordinated movement, demonstrating strength and coordination.
Once standing, the mare actively looked for food and water, and defecated and urinated normally. A urine sample was collected and found to have an increased specific gravity (1.055). No pigmenturia was noted. At a walk, the mare was extremely short-strided, and appeared to have very rigid movements. When standing still, hind limbs and forelimbs were close together, which gave the horse the appearance of a “horse-on-a-ball.” The tendons of the extensor muscles in the forelimbs were visible, and appeared tight. Both carpi were slightly flexed due to tendon contraction. The mare was reluctant to lift both hind limbs, and increased muscle tone was noted in anti-gravity extensor muscles. Increased muscle tone was also noted in the longissimus dorsi muscles on both sides. Muscle tone particularly in the hind limb musculature on both sides increased with the little exercise we forced on the horse to evaluate its gait, which consisted of a few meters of walking, turning, and circling. During this short walk the horse appeared well-coordinated, and ataxia was not noted. Digital pulses were not palpable on either limb before and after exercise. However, due to the previous suspicion of laminitis, radiographs were taken of all 4 distal limbs. These did not provide evidence for pedal bone rotation or sinking. An examination per rectum was performed to rule out a pelvic fracture or lumbo-sacral abnormalities, and was normal. The horse lay down normally, without evidence of previous muscle fasciculations or tremors. The musculature of her back and pelvis remained subjectively hypertonic even while at rest.
A complete blood cell count was normal with the exception of a mild hyperfibrinogenemia (14.7 μmol/L, normal: ≤ 11.8 μmol/L). A chemistry profile, which included enzymes creatine kinase (CK) and aspartate aminotransferase (AST), was normal. An IgM capture enzyme-linked immunosorbent assay (ELISA) testing for West Nile virus specific IgM antibodies (Veterinary Diagnostic Laboratories, CSU, Fort Collins, Colorado, USA) on a serum sample was negative, as was an immuno-fluorescent antibody test (IFAT) for Sarcocystis neurona-specific antibodies (Diagnostic Laboratory, UC Davis, California, USA), the organism causing equine protozoal myelo-encephalitis. A normal serum vitamin E concentration was measured at 1.76 g/L (normal: 1.00 to 3.00 g/L) (Veterinary Diagnostic Laboratories, CSU). We discussed the following steps with the owner: a cerebrospinal fluid collection, a muscle biopsy, and a potential referral to the Colorado State University Veterinary Teaching Hospital. However, the owner declined the procedures or transport mainly for financial reasons and stress involved with transportation. Instead, the owner opted to continue treatment with phenylbutazone and methocarbamol (Methocarbamol Tablets USP, West-Ward Pharmaceutical Corp., Eatontown, New Jersey, USA), 10 mg/kg body weight (BW), PO, BID.
Weekly updates provided by the owner did not show improvement. After 2 wk, a repeat visit revealed that the mare still had no major skin decubitus; however, the horse appeared more reluctant to even stay in a sternal position. The mare preferred to lie on her side and would eat and drink while sternal. According to the owner, the mare was able to rise for short durations, pass manure and urinate, and would then switch sides to lie down. We observed a moment of getting-up, followed by lying down within minutes. When recumbent, the musculature of the limbs were hard, and the neck musculature appeared now also involved. The upper limbs, when manipulated, were hypertonic on flexion and extension. During another 3 wk we repeated CK and AST activity measurements twice; these were consistently normal. We discontinued the administration of methocarbamol because of no noticeable change and because we were not convinced of a primary muscle problem. Instead, treatment with gabapentin (Gabapentin capsules; Caraco Pharmaceutical Laboratories, Detroit, Michigan, USA), 2.5 mg/kg BW, PO, BID was instituted, a drug that recently has been shown to bind to α2δ of voltage dependent calcium channel complexes, thereby limiting Ca++ influx in pre-synaptic terminals with an effect on neuropathic pain and excitation (5). However, a gradual deterioration of the mare’s condition rather than improvement or stabilization was noted over the next 2 wk. The mare was noted to lose body condition most likely due to decreased food intake and inactivity as the mare’s willingness and ability to rise decreased.
Differential diagnoses at this point included a myopathy, myotonia, collective pathology of the neuro-muscular junctions, and a diffuse or multifocal myelopathy that was thought to be extremely targeted to a select group of neurons. The mare’s status for hyperkalemic periodic paralysis (HYPP) was unknown, but HYPP did not fit with the presentation. Despite the lack of a muscle biopsy as an aid to a potential diagnosis, we felt that normal muscle-specific plasma enzyme activity made the diagnosis of a myopathy less likely, although not all myopathies result in increased enzyme activity. Neuro-muscular junction pathology causing a chronic, progressive hypertonia has not been described in horses. Weakness and muscular atrophy were not noticed which may have suggested equine motor neuron disease, chronic presentations of botulism, or other forms of lower motor neuron disease. Intoxication with organophosphates slows the degradation of the neurotransmitter acetylcholine, which is the main neuro-muscular junction neurotransmitter. Rigidity and hypertonia may have been a clinical sign of organophosphate intoxication; however, we would have expected to find more profound central nervous system signs such as seizures in addition to parasympathetic overstimulation.
We felt that the clinical picture resembled a mild form of tetanus; however, due to the chronicity of signs, lack of cranial nerve involvement, and a regular tetanus vaccination history, this diagnosis was unlikely. A cerebellar origin was briefly considered, but the idea was rejected due to a normal menace response, absence of an intention tremor, or a base-wide stance of the horse. It was thought that the observed clinical signs may have been the result of a toxin other than tetanus toxin affecting the spinal cord interneurons. Strychnine, which is a competitive antagonist of glycine, a neurotransmitter of interneurons, was briefly considered; however, at no point in time did we observe the violent tetanic seizures associated with strychnine intoxication, and we also would have expected some response to methocarbamol (6).
Ultimately, an immune-mediated or degenerative pathology to spinal cord interneurons was considered due to the symmetrical presentation of clinical signs and the absence of ataxia and weakness. However, distinct etiology or specific pathogenesis was not certain at that time. Treatment was initiated with dexamethasone [Dexamethasone (2 mg/mL) VetoneTM, MWI; Meridian, Idaho, USA], 80 mg IM once daily for 3 d, followed by 60 mg IM once daily for 3 d. Soon after the institution of this treatment, the owner reported improvement in the horse. After the first 3 injections the horse was standing and ambulating for more than 50% of the time. Then, after 3 more days of treatment, the owner described a horse that was standing and walking around for more than 75% of a 24-hour period. We felt encouraged that the treatment with dexamethasone was effective, which led to further review of the current literature. We took note of “stiff-horse syndrome,” described by Nollet et al (4), where a horse is described with unexplainable stiffness and an increased glutamate decarboxylase antibody titer, suggesting a depletion of GABA at the interneuronal junctions.
A serum sample obtained from the mare prior to injection of dexamethasone was submitted to a laboratory (ARUP Laboratories, Salt Lake City, Utah, USA), which provided routine testing for GAD antibody concentration in humans using a radioimmunoassay (RIA) (GAD65 RIA kit, Kronus, Star, Idaho, USA). An age, breed, and gender-matched serum sample was also submitted as a control. Results revealed an increased GAD antibody concentration in the mare [2.71 U/L, (human normal: 1.0 to 1.5 U/L)], and a concentration < 1.0 U/L in the control serum. The mare showed gradual clinical improvement with continued low-dose dexamethasone administration (lowest dose: 8 mg IM every other day). The mare became asymptomatic by December 2008, and the dexamethasone administration was discontinued.
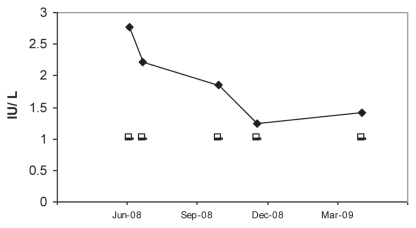
Figure 1 shows the decreasing GAD antibody concentration over time and compared to the mean GAD antibody concentration from 5 control samples obtained from age-, breed-, and gender-matched horses. Each time a serum sample from the clinically affected mare was submitted a serum sample from a control horse was also submitted. After regression of clinical signs in the mare GAD antibody activity in the mare was monitored as an early indicator of clinical disease reoccurrence. A repeat sample was obtained 3 mo after discontinuing dexamethasone administration. Figure 1 reveals that the GAD antibody concentration had increased from 1.21 IU/L (Dec 08) to 1.44 IU/L (March 09) with the mare still being asymptomatic. However, in May 2009, the owner reported that the mare was more frequently recumbent again, and once standing, exhibited the same clinical signs as described previously. During a visit we concurred, and a serum sample was obtained and treatment was re-initiated with dexamethasone. The GAD result on this sample sent to the laboratory was negative. Because of this finding, previously collected samples from the mare which had been stored frozen were sent to the laboratory for analysis. This time all samples were negative for GAD antibody activity. The laboratory reported that it had changed the testing method from a radioimmunoassay (RIA) to an enzyme-linked immunosorbent assay (ELISA). Both assays, produced by the same company, use the same GAD65 antibody, and therefore we concluded that testing horse samples with this ELISA kit is inappropriate and likely to cause false-negative test results.
Figure 1.
Serum GAD antibody concentration (U/L) determined on 5 occasions in the patient with generalized stiffness and prolonged recumbence (filled diamonds), and serum GAD antibody concentrations of 5 age, breed, and gender-matched, asymptomatic horses (control, open squares). A patient sample was paired each time with a single control sample and sent for analysis. Results of the control samples were combined and reported as a mean concentration. Four of 5 control samples were reported as “< 1 U/L.” For calculation of the mean 1 U/L was used. The test implemented was a radioimmunoassay (GAD65 RIA kit; Kronus, Star, Idaho, USA) with reported normal results in humans as 1 to 1.5 U/L.
The mare was treated again with dexamethasone, recovered quickly and is currently asymptomatic and under saddle. The duration of treatment with dexamethasone for this second episode was shortened to 7 wk. We warned the owner that re-occurrence of the syndrome is likely.
Discussion
Stiff-person syndrome is a rare, immune-mediated disease in humans. It causes intermittent muscle hypertonia and stiffness due to increased GAD antibody activity. Cerebellar ataxia has also been described in humans with increased GAD antibody titers (7). Human diabetes mellitus type I (DM-I) may also result in an increased GAD antibody titer. Mainstay therapy in humans is plasma exchange, and diazepam and phenobarbital therapy. In our equine case we were unable to find a reason for GAD antibody presence. While DM-I is extremely rare in horses, DM type II is quite frequent and is often associated with obesity. Human DM-II patients do not exhibit increased GAD antibody titers. Interestingly, a case report suggested a relationship between a West Nile virus infection (WNV) and a case of human SPS based on the timely occurrence of the 2 diseases, and due to similarities between antigenic epitopes of GAD and WNV (8). However, WNV is endemic to the United States, and we do not currently perceive an increase in SHS (or SPS) cases in northern Colorado.
The owner of this mare is prepared for reoccurrence of the problem, which, in the case of future relapses, should respond to corticosteroid administration. We are currently comparing 2 strategies of corticosteroid administration. The first course of treatment focused on a high initial dose of dexamethasone (80 mg IM once daily) over several days, then gradually decreasing the dose of dexamethasone over months to a low dose (as low as 8 mg IM every other day). We initiated this treatment in June 2008, and continued until December 2008. With reoccurrence of clinical signs the second time, we initiated corticosteroid therapy with 40 mg dexamethasone once daily for 5 d, and then tapered the dose to 10 mg every other day for another 6 wk with no significant differences in disappearance of clinical signs. Immunosuppressive doses of dexamethasone are indicated to suppress antibody production by plasma cells, and a prolonged “low dose treatment schedule” of dexamethasone should not be sufficient to achieve this goal. In this particular case it will be important to monitor for potential reoccurrence of clinical signs. The interval between discontinuation of corticosteroid therapy and reoccurrence of clinical signs will largely determine future dosing schedules in this mare.
Addendum
As a follow-up on this mare, clinical signs of stiffness and recumbency (noted previously), recurred every 8 to 15 mo since 2008 during an additional observation period [2008 — (mid) 2011]. Clinical signs occurred abruptly and were noted by the owner and confirmed by one of the authors (ADS, LSG). Recurrence of clinical signs has been limited to winter or spring months. A low-dose dexamethasone therapy (starting with 30 mg dexamethasone IM injections q24h for 7 d, then decreasing to 15 mg IM q24h over a period of 3 to 4 wk) provides rapid and complete disappearance of clinical signs. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.van Galen G, Delguste C, Sandersen C, et al. Tetanus in the equine species: A retrospective study of 31 cases. Tijdschr Diergeneeskd. 2008;133:512–517. [PubMed] [Google Scholar]
- 2.Furr M. Clostridial neurotoxins: Botulism and tetanus. In: Furr M, Reed S, editors. Equine Neurology. Ames, Iowa: Blackwell Publ; 2008. pp. 221–229. [Google Scholar]
- 3.Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: Diagnostic clues for this association. Brain. 2008;131:2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 4.Nollet H, Vanderstraeten G, Sustronck B, et al. Suspected case of stiff-horse syndrome. Vet Rec. 2000;146:282–284. doi: 10.1136/vr.146.10.282. [DOI] [PubMed] [Google Scholar]
- 5.Davis JL, Posner LP, Elce Y. Gabapentin for the treatment of neuropathic pain in a pregnant horse. J Am Vet Med Assoc. 2007;231:755–758. doi: 10.2460/javma.231.5.755. [DOI] [PubMed] [Google Scholar]
- 6.Furr M. In: Equine neurotoxic agents and conditions. Equine Neurology. Furr M, Reed S, editors. Ames, Iowa: Blackwell Publ; 2008. pp. 337–356. [Google Scholar]
- 7.Honnorat J, Saiz A, Giometto B, et al. Cerebellar ataxia with antiglutamic acid decarboxylase antibodies: Study of 14 patients. Arch Neurol. 2001;58:225–230. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- 8.Hassin-Baer S, Kirson ED, Shulman L, et al. Stiff-person syndrome following West Nile fever. Arch Neurol. 2004;61:938–941. doi: 10.1001/archneur.61.6.938. [DOI] [PubMed] [Google Scholar]